Table of Contents
Advertisement
General
Indications for Use Statement for US FDA
Regulatory remarks
Importer Information
1-10
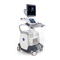
Voluson™ SWIFT / Voluson SWIFT+ are a general-purpose diagnostic ultrasound system
intended for use by a qualified and trained healthcare professional for ultrasound imaging,
measurement, display and analysis of the human body and fluid. Voluson™ SWIFT / Voluson
SWIFT+ clinical applications include: Fetal/Obstetrics; Abdominal (including renal and GYN/
Pelvic); Pediatric; Small Organ (Breast, Testes, Thyroid, etc.); Neonatal Cephalic; Adult
Cephalic; Cardiac (Adult and Pediatric); Peripheral Vascular (PV); Musculo-skeletal
Conventional and Superficial; Transrectal (including Urology/Prostate) (TR); Transvaginal
(TV).
Mode of operation include: B, M, PW Doppler, CW Doppler, Color Doppler, Color M Doppler,
Power Doppler, Harmonic Imaging, Coded Pulse, 3D/4D Imaging mode, Elastography and
Combined modes: B/M, B/Color, B/PWD, B/Power/PWD. The Voluson™ SWIFT / Voluson
SWIFT+ are intended to be used in a hospital or medical clinic.
•
First CE marked in 2020.
•
Federal law restricts this device to sale by or on the order of a physician!
•
This machine must be used in compliance with the law. Some jurisdictions restrict certain
uses such as gender determination, contrast imaging, IVF, PUBS or CVS, etc. Please
consider the local laws and regulations.
•
The equipment conforms with regulations for electrical safety IEC 60601 and safety class
IIa according to the MDD 93/42/EEC regulation for use on human patients.
The manufacturer, assembler, importer or installer consider themselves responsible regarding
safety, reliability and performance of the equipment under the following conditions:
•
Authorized personnel has performed installation and initial start-up of the system.
•
Options or new settings have only been added by authorized personnel.
•
Authorized personnel has performed modifications or repairs.
•
The local electric installation complies with the national regulations.
•
The equipment is only used according to the Instructions for Use.
•
Paper copy : The EU commission Regulation on electronic instructions for use of medical
devices in the European Union demands, that a paper copy of Instructions for Use can
be ordered at no additional charge. You may therefore, send a request to
volusondocumentation-request@ge.com. This request will be treated within 7 days.
•
Türkiye İthalatçısı
GE Medical Systems Türkiye Ltd. Şti.
Esentepe Mah. Harman Sok. No: 8
34394 Şişli İstanbul Türkiye
Voluson™ SWIFT / Voluson SWIFT+ Instructions For Use
5831612-100 Revision 4
- Quick Links:
- Table of Contents
Hide quick links:
Advertisement
Table of Contents














Need help?
Do you have a question about the Voluson Swift and is the answer not in the manual?