Table of Contents
Advertisement
g
GE Medical Systems
Quality Assurance Testing
Purpose:
Customer Name:
Address:
System ID #:
Purchase Order #:
System Configuration
Video Cassette Recorder
Line Printer
Video Page Printer
Urological Therapy Guidance
Other
___________________________________
_________________________________________
Field Service Engineer: ____________________________________
TEST EQUIPMENT
NAME
Leakage Tester
Multimeter
Gray Scale Phantom
TRANSDUCERS TESTED
The two transducers used most frequently should be listed as transducer number 1 and 2. Use the
separate documents provided to document the remaining transducers.
Transducer 1
Model: ________________________
Type:
General Purpose
Endorectal
Freq:
2-3.5 MHz
Scan Format:
Transducer 2
Model: ________________________
Type:
General Purpose
Endorectal
Freq:
2-3.5 MHz
Scan Format:
US Unit Manufacturer:
Samsung GE Medical Systems
Biopsy Guide
Spectral Doppler
Color
MANUFACTURER
3-5 MHz
Phased Array
Mechanical
3-5 MHz
Phased Array
Mechanical
1/16
Refer to the Ultrasound QA Reference
Manual 2262684-100 for details.
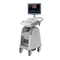
System Model:
LOGIQ a200
System Serial #:
Dispatch #:
System Status:
Configuration Notes:
MODEL
Atten.
Serial Number: ______________________________
Superficial
Endovaginal
5-7.5 MHz
Linear Array
Curved Linear Array
Other _____________________
Serial Number: ______________________________
Superficial
Endovaginal
5-7.5 MHz
Linear Array
Curved Linear Array
Other _____________________
2277615-100
REV 0
Survey Date:
Year of Mfg.:
Warranty
Contract
Employee #: _______________
SERIAL #
CAL DATE
Intraoperative
Other _____________________
7.5 MHz and higher
Intraoperative
Other _____________________
7.5 MHz and higher
Billable
Hide quick links:
Advertisement
Table of Contents