Table of Contents
Advertisement
INTENDED USE
The iVent™201 MR Conditional is a portable, computer controlled, electrically
powered intensive care ventilator intended to provide continuous or intermittent
ventilatory support for the care of individuals who require mechanical ventilation.
Specifically, the ventilator is applicable for use with adult through pediatric
patients, who require invasive or non-invasive assistance via the following
general modes of ventilatory support, as prescribed by an attending physician:
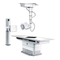
The iVent™201 MR Conditional ventilator is suitable for use in the ICU and all
other hospital areas, including Magnetic Resonance (MR) environment, not to
exceed a 3.0 Tesla static magnetic field, in all hospital-type facilities, alternate
care sites, transport, emergency and in the home environment. The iVent™201
MR Conditional ventilator is MR conditional.
The optional non-invasive Pulse Oximeter is intended for non-invasive monitoring
of oxygen saturation and pulse rate and is suitable for use in all above mentioned
areas, excluding MR environments.
The iVent™201 MR Conditional ventilator is a restricted medical device intended
for use by qualified, trained personnel under the direct supervision of a physician.
USE OF THE IVENT
The iVent™201 ventilator (serial number 15 000 and higher) is classified as MR
conditional for 1.5T and 3T MR scanners. This means that the iVent
use in the MR environment if the operation conditions specified in this section are
met.
For the MRI conditional specifications refer to MRI Conditional Tests
Specifications, on page 29 of this document.
CAUTION
NOTE
Assist/Control (Pressure Controlled or Volume Controlled)
SIMV (Pressure Controlled or Volume Controlled)
CPAP/PSV
201 WITH MRI
TM
The iVent
201 ventilator should not be subjected to field strength
TM
greater than 100 Gauss and must be kept outside of 100 Gauss
perimeter.
The iVent
201 MR Conditional has been demonstrated to pose no known
TM
hazards in MR Environment under specified conditions of use. However, MR
image quality may degrade when working with the ventilator in a specific MR
suite. It is recommended to perform a pre-qualification image quality test to
ensure an acceptable level of image quality. For more details contact your
local sales representative
25
1 Introduction
201 is safe to
TM
Advertisement
Table of Contents