Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for promotal ELOI-PODOLOGIE Apolium 2800 Series
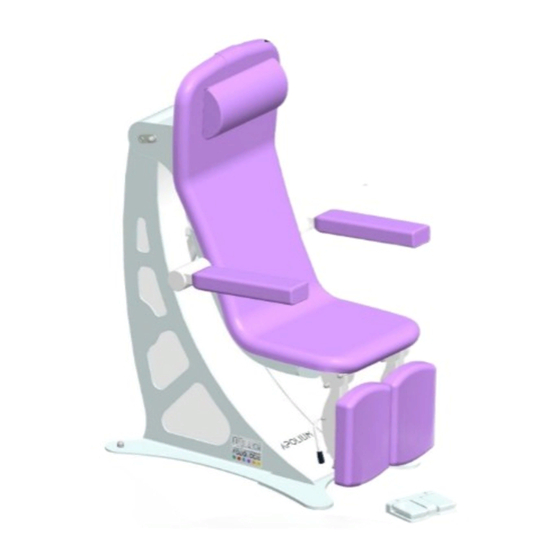
- Page 1 User Manual Model presented: 2800-10 PODIATRY chair 2800-10 / 2800-20 / 2800-30...
- Page 2 User Manual are considered to be correct at the time of 22, rue de Saint-Denis de Gastines printing. B.P. 26 - 53500 ERNÉE Cedex Promotal reserves the right to modify or declare obsolete its FRANCE models and procedures without notice. Tel.:...
-
Page 3: Table Of Contents
Summary Important information Safety symbols Applied parts Electrical power supply Electromagnetic interference Unpacking precautions Medical device delivered on a wooden pallet Storage conditions Conditions of use Unpacking and Installation Step by step Check Remark: Authorised EC Representative Cleaning protocol Warning Cleaning/Disinfecting User manual –... - Page 4 Summary (reference 167-10) Installing the roll holder Accessories Installing the paper roll Plastic cover (head end) Installing the protective cover Head rest Installing the head rest Positioning Adjustment Rectangular pan Positioning “Kidney dish” pan Positioning Pan for plastic bag Positioning Plastic bag Disposable cover / leg rest Positioning...
-
Page 5: Important Information
Important information Safety symbols Warning sign Information marked with this symbol must be read and strictly complied with! Remark Draws your attention to a procedure, practice or situation. Maximum number of Correct orientation for Humidity limitation stacked pallets transportation Atmospheric pressure Temperature Fragile limitation... -
Page 6: Applied Parts
Electromagnetic interference This Promotal medical device was designed and built to minimise electromagnetic interference with other equipment. If interference is, however, observed, remove the apparatus causing the interference from the room and/or plug it into an isolated circuit. -
Page 7: Unpacking Precautions
Unpacking precautions Medical device delivered on a wooden pallet The medical device positioned on a wooden pallet ensure that the forklift truck is correctly positioned may be easily moved using a forklift truck, as long in relation to the pallet, and that the unit is stable. as this is used correctly. -
Page 8: Check
2) Check the external components for any Remark: Authorised EC Representative Within the European Union, all problems, complaints or questions should be addressed to the Authorised EC Representative of Promotal indicated below: Promotal 22, rue de Saint-Denis de Gastines 53500 Ernée,... -
Page 9: Cleaning Protocol
• The use of abrasive powders or any other abrasive product should be avoided. • High-pressure cleaning is forbidden. Under no circumstances shall Promotal be held liable under warranty for any damage caused by non-compliance with the use instructions for a detergent- disinfectant. -
Page 10: User Manual - Apolium
User manual – APOLIUM Intended use This Medical Device is designed for use in professional premises only: • Podiatry or Pedicure Medical Practice. • Healthcare establishments This appliance must not be installed in domestic premises. This Medical Device is intended for podiatry and pedicure medical examinations and acts. -
Page 11: Electrical Connection
User manual – APOLIUM Electrical connection This medical device must be connected to the mains supply. • Connection to the mains supply: 120 V or 230 V (depending on the country) • Frequency: 50/60 Hz • Protection classification: Class 2/ type B device •... -
Page 12: Safety
Safety Caution To avoid malfunctions and for safety reasons, no objects must be left under the chair's seat or between its moving frames. Caution the supply cable for this medical device may represent an obstacle and cause falls. Do not forget its presence when moving around the device once connected to the mains supply. - Page 13 Safety note Before using the medical device lowering control, ensure that no objects or obstacles are between the moving parts and the ground. Safety note When using pre-programmed positions ((reception, QE1) and QE2 for the 2800-30 model), all movements can be stopped by simply pressing the buttons on the foot pedal or, as a last resort, by disconnecting the mains plug.
-
Page 14: Using The Individual Control
Using the individual control The programmed functions are grouped on manual command and the foot pedal. Manual command (for model 2800-30) Presentation Safety note When using pre-programmed positions (reception, QE1, QE2), do not leave the patient on the chair unattended. QE = Quick Exam Setting the height (Down) Setting the height using press-and-hold... -
Page 15: Foot Pedal
The programmed functions are grouped on the foot pedal. Foot pedal (for model 2800-30) Presentation Safety note When using pre-programmed positions (reception, QE1, QE2), do not leave the patient on the chair unattended. QE = Quick Exam QE2 position QE1 position Position fully Position fully configurable by... -
Page 16: Manual Command
Using the individual control The programmed functions are grouped on the manual command and the foot pedal. Manual command (for models 2800-10 AND 2800-20) Presentation Nota sécurité Lors du rappel de positions programmées (accueil et examen), ne pas laisser le patient installé sans surveillance sur le fauteuil. Using the foot pedal Just press and hold the foot pedal’s push button to activate the desired movement. -
Page 17: Foot Pedal
The programmed functions are grouped on the foot pedal. Foot pedal (for models 2800-10 AND 2800-20) Presentation Safety note When using pre-programmed positions (reception and examination), do not leave the patient on the chair unattended. Using the foot pedal Just press and hold the foot pedal's push button to activate the desired movement. The movement stops as soon as you take your foot off the pedal. -
Page 18: Using The Chair
Using the chair Adjusting the height Using the foot pedal Caution Make sure their are no objects under the chair before lowering it. Height safety mechanism When returning to the reception position chair stops at 55cm. Release the pedal, then press on the button used to lower the chair to continue lowering it down to 50cm. - Page 19 Setting the tilt angle of the leg rest (for model 2800-10) This equipment is fitted with a mechanism that locks the leg rest permanently. To change the tilt upwards, grip the lower part of the leg rest and raise it to the required tilt. To change the tilt downwards, hold the leg rest with one hand and push the control lever (L2) of the locking system downwards with the other.
- Page 20 Using the chair Setting the tilt angle of the leg rest (for model 2800-30) Setting the leg plate tilt RIGHT Setting the leg plate tilt LEFT All the individual controls are used by pressing and holding. (releasing the control interrupts the corresponding movement). Safety To avoid malfunctions and for safety reasons, no objects must be left under the leg rest or between the chair's moving...
- Page 21 Setting the tilt angle of the leg rest Setting the length of the leg rest Caution When adjusting the leg rest, lift the patient's leg up slightly to take the weight off the leg rest. User Manual...
-
Page 22: The Levellers
Using the chair The levellers Adjusting the levellers Screw or unscrew the leveller (P) to the required height. Paper roll (reference 167-10) Installing the roll holder Allen key n°4 User Manual... -
Page 23: Accessories
Only accessories designed and provided by Accessories Promotal for this medical device are authorised for use. Installing the paper roll Caution Take care to follow the instructions Unroll the paper onto the upholstery before the order maximise patient gets onto the chair. -
Page 24: Head Rest
Only accessories designed and provided by Accessories Promotal for this medical device are authorised for use. Head rest (reference 2802-01) Installing the head rest Positioning Push the head rest's band insert into the plate fixed to the back rest. Adjustment Just press on the head rest to lower it, or pull on the band insert to raise it. -
Page 25: Rectangular Pan
Only accessories designed and provided by Promotal for this medical device are authorised for use. Rectangular pan (reference 2864-01) Positioning “Kidney dish” pan (reference 2864-10) Positioning User Manual... -
Page 26: Pan For Plastic Bag
Only accessories designed and provided by Accessories Promotal for this medical device are authorised for use. Pan for plastic bag (reference 2874-01) Positioning Plastic bag (reference 962) Delivered in rolls of 20 bags. User Manual... -
Page 27: Disposable Cover / Leg Rest
Only accessories designed and provided by Promotal for this medical device are authorised for use. Disposable cover / leg rest (reference 2804-01) Delivered in boxes of 100 items. Positioning User Manual... -
Page 28: Options
Options Back support (reference 2880-01) Adjustment The back support cushion is inflated using a manual pump (pneumatic bulb). The cushion is deflated by pressing on the valve (located close to the bulb) while pressing down with the lower back. User Manual... -
Page 29: Accumulator
Accumulator (reference 2892-01) Accumulator - technical characteristics Voltage ......: 24 V DC Amperage ......: 2 Ah Protection rating ....: IP66 Accumulator type ....: Lead gel Voltage (maximum) ... : 30 V DC Charging time ....: Approx. -
Page 30: Medical Device Lifetime
Medical device end of service life Your dealer is responsible for the recovery and end of life treatment of this device. If necessary, do not hesitate to contact Promotal. We can propose solutions to treat this equipment in the best conditions. -
Page 31: Replacing Used Batteries In The Foot Pedal
Replacing used batteries in the foot pedal (battery reference (x2): LR03 - AAA Alkaline 1.5V) To replace the batteries in this wireless foot pedal: Turn the pedal over Loosen the screw and remove the battery compartment cover Remove the two used batteries and replace them with the two new batteries. (make sure to place them with their polarities correctly positioned.) Replace the cover and tighten the screw. -
Page 32: Maintenance Notebook
Maintenance notebook User Manual... -
Page 33: Notes
Notes User Manual... -
Page 34: Warranty Information
Commitments Promotal undertakes to replace defective parts returned during the warranty period and which are found to be defective after examination by Promotal. The distributor of Promotal is responsible for after sales service during and after the warranty period. - Page 35 22, rue de Saint-Denis de Gastines, 53500 Ernée - FRANCE Tél. : +33 (0)2 43 05 17 76, Fax : + 33 (0)2 43 05 72 00, www.promotal.com S.A.S. au capital de 4 290 660 € - R.C.S. LAVAL Siret 421 156 720 00011 - TVA FR 67 421 156 720 - APE 3250A...
- Page 36 PROMOTAL - FRANCE www.promotal.com DIC2800-10_3617EN...
















Need help?
Do you have a question about the ELOI-PODOLOGIE Apolium 2800 Series and is the answer not in the manual?
Questions and answers