Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

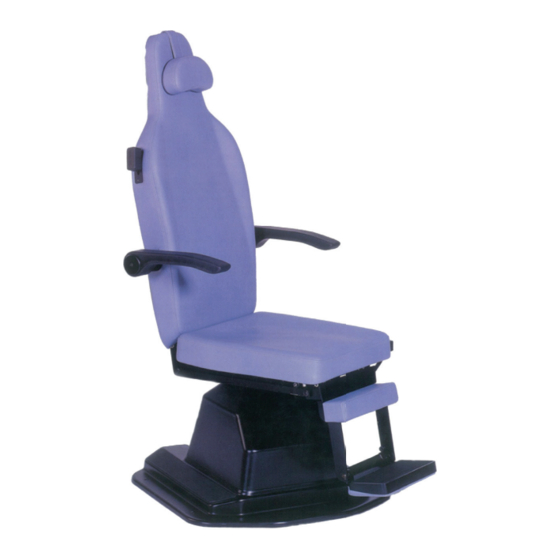
Summary of Contents for Atmos Patient chair E1
- Page 1 MedizinTechnik English ATMOS Patient chair E1 503.0450.B 2010-07 Index: 03 ATMOS MedizinTechnik Ludwig-Kegel-Straße 12, 14-16, 18 Tel. +49 (0) 7653 / 689-0 atmos@atmosmed.de 79853 Lenzkirch/Germany GmbH & Co. KG Fax +49 (0) 7653 / 689-190 www.atmosmed.de...
-
Page 2: Table Of Contents
Notes on EMC ............18-20 12.0 Declaration of Conformity General tems and conditions Further information, accessories, consumables and spare parts are available from: ATMOS MedizinTechnik GmbH & Co. KG Ludwig-Kegel-Straße 16 79853 Lenzkirch Deutschland / Germany Tel. +49 (0) 76 53 / 689-0... -
Page 3: Introduction
These operating instructions must always be kept available near the device. Care and safety inspections in conjunction with professional execution provide for operational safety and readiness for use of your ATMOS Patient chair E1 and are therefore a must besides regular cleaning. -
Page 4: Explanation Of Pictures And Symbols
Symbols within these operating instructions Warning, Important notes especial diligent notice! Symbols of the ATMOS patient chair E 1 Application part type B Order number The CE sign shows that this product meets the appropriate requirements of guidelines. -
Page 5: Scope Of Supply
Scope of supply ● Prior to dispatch, the ATMOS patient chair E1 was subjected to an extensive functional test and has been carefully packed. Nevertheless, please compare the contents of the shipment on completeness immediately upon receipt (see delivery note). -
Page 6: For Your Safety
• The ambient conditions specifi ed in section 9.0 "Technical specifi cations" must be strictly observed. The ATMOS patient chair E1 is not intended for operation • in areas of medical rooms subject to danger of explosion. Areas subject to explosion hazards may be created by the use of infl ammable anaesthetics, skin cleansers and disin- fectants. -
Page 7: Setting Up And Starting Up
Setting up and starting up Setting up Set up the device on a level, fi rm surface. Mains voltage and fuses: Mains voltage: 230V / 50Hz (120V/60Hz) Fuses: Thermal fuse: 3 A (230V) Thermal fuse: 6 A (120V) Starting up ●... -
Page 8: Fastening The Chair
The chair is not destined for use in explosion- hazardous areas. Power connection The patient chair E1 is delivered with an IEC power connector. It is inserted into the IEC power socket on the rear of the base and linked with a properly installed earthing contact socket. -
Page 9: Operating
= downwards The Patient chair E1 is additionally equipped with an "automa- tic down"-function so that the upper part of the chair can be adjusted to its lowest position by a short movement with the pedal regulator (fi g. -
Page 10: Adjusting The Backrest
Operating Adjusting the backrest – Press lever element ( , fi g. 2) downwards, – adjust backrest to the desired position, – release lever element which will then return to its initial position, – backrest and foot support are arrested. ... -
Page 11: Adjusting The Head Rest
Operating Adjusting the head rest The headrest can be adjusted to a lower position by simply pulling out the retaining band; a higher position is reached by pushing the band in. For removing the headrest, pull the retaining band completely out of the backrest. Fig 4 Head rest Foot rest If required, the foot rest can be folded down (fi g. -
Page 12: Cleaning
If liquid has penetrated the unit, it may not be operated again until it has been checked by the authorised customer service centre. ● The surfaces of the ATMOS patient chair E1 are resistant against all the surface disinfectants. Nevertheless after any length of time discolourations could possibly develop. -
Page 13: Recommended Surface Disinfectants
Cleaning 5.3 Recommended surface disinfectants The following surface disinfectants were tested on their suitability: Disinfectant Contents (in 100g) Manufacturer TERRALIN Benzalkonium chloride 20,0 g Schülke & Mayr, Norderstedt Phenoxypropanoles 35,0 g (Application concentrate) Didecyl dimethyl 14,0 g ammonium chloride 10,0 g QUATOHEX Benzalkonium chloride 07,5 g... -
Page 14: Maintenance And Service
Chair does not start up – No mains voltage – Check house fuse – Check mains plug on perfect fi t at the device – Defective fuse – Exchange the fuse In the case that any malfunction, please immediately inform your authorised ATMOS service. -
Page 15: Accessories And Spare Parts
Accessories and Spare Parts 8.1 Accessories Child seat on request 8.3 Spare parts Upper part, complete ..........070.0001.0 Base, complete, 220V ..........070.0002.0 Base, complete, 127V ..........070.0002.1 Base plate............... 070.0003.0 Covering, complete..........070.0004.0 Foot switch, complete ..........070.0005.0 Calf cushion ............070.0006.0 Plate for foot-rest, incl. -
Page 16: Technical Specifi Cations
Technical specifi cations Voltage 230 V~ ± 10 %; 50 Hz Special voltage: 120 V~ ± 10 %; 60 Hz Current input 2,4 A (230 V~) 4,6 A (120 V~) Power consumption 550 VA Fuses Thermal fuse....3 A (230V) Thermal fuse....6 A (120V) Operating time Max. -
Page 17: Disposal
10.0 Disposal The ATMOS patient chair E1 does not contain any hazardous materials. ● The housing is recyclable. ● Device and accessories must be decontaminated prior to disposal. ● Please take care on a careful separation of the different materials. -
Page 18: Notes On Emc
12.1 Guidelines and Manufacturer´s Declaration - Emissions The ATMOS Patient chair E1 is intended for use in the electromagnetic environment specifi ed below. The customer or user of the ATMOS Patient chair E1 should ensure that it is used in such an environment. - Page 19 12.3 Guidelines and Manufacturer´s Declaration - Immunity The ATMOS Patient chair E1 is intended for use in the electromagnetic environment specified below. The customer or user of the ATMOS Patient chair E1 should ensure that it is used in such an environment.
- Page 20 If the measured field strength at the location where the ATMOS Patient chair E1 is used exceeds the above compliance level, the ATMOS Patient chair E1 is to be observed to verify the intended use. If abnormal performance characteristics are noted, additional measures might be necessary, e. g. a changed arrangement or another location for the device.
-
Page 21: Declaration Of Conformity
12.0 Declaration of Conformity... - Page 22 ATMOS MedizinTechnik GmbH & Co. KG in accepting such goods or payment arrears. Acts of God or stoppages upon in writing by us.













Need help?
Do you have a question about the Patient chair E1 and is the answer not in the manual?
Questions and answers