Table of Contents
Advertisement
Advertisement
Table of Contents
Troubleshooting

Subscribe to Our Youtube Channel
Summary of Contents for Breas Clearway 2
- Page 1 User Manual 009077 V6 Revised: MAR 2022...
-
Page 2: Table Of Contents
Preparing the Clearway 2 for Use ................. 25 Checking the Device before Use ..............25 Placing the Clearway 2 Ready for Use ............25 Connecting to AC Power (Mains Power) ............26 Inspecting the Clearway 2 before Use ............26 How to Use the Clearway 2 ................... - Page 3 Environmental Operating Conditions .............. 69 Dimensions ....................70 Emissions and Immunity Declaration ............. 71 Compliance of Standards ................72 Clearway 2 in other countries ................. 77 Accessories ......................78 Package Contents ..................78 Optional Accessories ................79 Patient Settings ......................... 80 Glossary of Terms ......................
-
Page 4: Introduction
Clearway 2. Breas Medical Ltd reserves the right to make changes to this product without any prior notification. U.S. Federal law restricts this device to sale by or on order of a physician. -
Page 5: Icons
Risk of equipment damage, loss of data, extra work, or unexpected results Note Information that may be valuable but is not critically important Reference Reference to other manuals with additional information on a specific topic Introduction Clearway 2 User Manual DOC-001000 Ver 6... -
Page 6: What Is The Clearway 2
• Intermittent Positive Pressure Breathing (IPPB) • Non-Invasive Ventilation (NIV) for therapeutic use Intended Use Only use the Clearway 2 as it is intended. Failure to do so may cause serious harm or injury to the patient. Never use without the appropriate training and resuscitation equipment available. - Page 7 Individuals who may benefit from the use of the Clearway 2 include any patient with an ineffective cough due to; • Muscular Dystrophy •...
- Page 8 • Emphysema • Post-surgical patients (with no contraindications) The Clearway 2 device can be used for MI-E in both invasively and non- invasively ventilated patients as well as patients who are self-ventilating. The Clearway 2 device may be used either with a facemask, mouthpiece, or with a suitable adapter to a patient’s endotracheal (ET) or tracheostomy tube (MI-E...
-
Page 9: Warnings & Cautions
• Monitor the device while in use and stop providing treatment if the device malfunctions • The Clearway 2 must only be serviced by personnel certified by Breas • Use only power cords supplied by Breas Medical Ltd for this device •... - Page 10 Cautions! • Always place the Clearway 2 on a hard, clean surface, not on the floor • Always place foot pedal on dry surface. Ensure cable does not cause a trip hazard • Always position the Clearway 2 so that the air inlets are not obscured or blocked •...
-
Page 11: Contraindications To Use
The patient should be reviewed regularly to ensure that their condition has not altered. If the user of the Clearway 2 develops one of the below contraindications, contact your referring Clinician before continuing to use the Clearway 2: •... -
Page 12: Considerations Before Use
• Cardiovascular instability • Flail chest Considerations before Use Do not use the Clearway 2 with patients with the below conditions without specific instructions from your referring clinician. A risk assessment and guidance should be provided. If the patient develops any of the below symptoms/conditions, you must consult the referring clinician before continuing use of Clearway 2. -
Page 13: About This Manual
This manual is intended for the lay caregiver of a patient requiring medical use of the Clearway 2 device. The manual comprises information on general use of the Clearway 2. Patients and lay caregivers may read this manual for reference purposes, after appropriate guidance from the responsible care provider. -
Page 14: Safety Information
Clearway 2 device for either MI-E or IPPB. • Do not use the Clearway 2 in the event of suspected damage to the device, unexplainable or sudden pressure, performance or sound changes during operation, or if the delivered air from the Clearway 2 is abnormally hot or emits an odour. -
Page 15: Electrical Safety
Electrical Safety To ensure electrical safety is maintained ensure the following procedures are met: • Do not operate the Clearway 2 if it has a damaged power cord or casing. • To avoid electrical shock, disconnect the electrical supply to the Clearway 2 before cleaning. -
Page 16: Environmental Safety
• Do not expose the Clearway 2 to rain or snowfall. • Do not use or store in the presence of strong electromagnetic fields such as an MRI environment. Use of the Clearway 2 in an MR environment may result in malfunction of the Clearway 2 and pose unacceptable risk to the patient, medical staff, or other persons. - Page 17 • Do not use the Clearway 2 positioned in a warm place, such as direct sunlight or close to a radiator. • Do not use the Clearway 2 positioned near an open fire. • The device complies with the EMC requirements of standards listed in “Compliance of Standards”...
-
Page 18: The Usage Of Oxygen If Prescribed
• Ventilate the room adequately. • Do not smoke in a room where oxygen is being used. • Do not use the Clearway 2 positioned near or in a room with an open fire. • Naked light bulbs and other sources of ignition must be kept a minimum of 6 feet (2 meters) away from the oxygen cylinder or any part of the patient circuit. -
Page 19: Commonly Used Abbreviations And Terms
A single run of a set number of insufflations, followed by an Exsufflation (unless used in NIV or IPPB) Treatment A treatment is a set number of cycles of the prescribed settings, at the frequency stated by the prescribing physician/physiotherapist/doctor Safety Information Clearway 2 User Manual DOC-001000 Ver 6... -
Page 20: Product Description
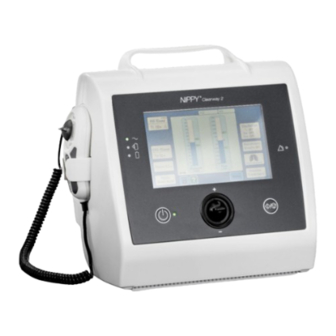
3 Product Description Main Components The main components of Clearway 2 are detailed below: Description Product Code Clearway 2 Device 232000 Clearway 2 Carry Bag 008284 AC Mains Power Cable 009074 User Manual 009077 Patient MI-E Circuit 008276 For more accessories please see section 10, Accessories... -
Page 21: Front Panel
Front Panel Symbol Description Standby ON/OFF Green Start/Stop Treatment (None) AC Power Source Green External Battery Green Internal Battery Green Alarm Amber Product Description Clearway 2 User Manual DOC-001000 Ver 6... -
Page 22: Side Panels
SD Memory Card slot is restricted to be used to save patient compliance data to a memory card, only. Back Panel External Power is restricted to be used only to power the device using an external battery Product Description Clearway 2 User Manual DOC-001000 Ver 6... -
Page 23: Equipment Designation And Safety Labels
Alarm Ingress protection rating against solids and liquids IP 22 Meets all requirements for CE marking according to applicable European health, safety and environmental protection legislation Product Description Clearway 2 User Manual DOC-001000 Ver 6... - Page 24 Product shall be kept away from rain and in dry conditions. The product or its material is part of a recovery or recycling process Product Description Clearway 2 User Manual DOC-001000 Ver 6...
-
Page 25: Preparing The Clearway 2 For Use
• Make sure that nothing can block the patient air inlets. • Do not place the Clearway 2 on a soft, or dusty surface, as this could make the device unstable, or result in dusty air to be drawn into the device. -
Page 26: Connecting To Ac Power (Mains Power)
• Check that the cables are properly connected. Inspection of Placement • The Clearway 2 shall be placed on solid flat surface below the patient level. (See “Placing the Clearway 2 Ready for Use” on page 25.) • Make sure that nothing can block the air inlets on rear and side of the device. -
Page 27: How To Use The Clearway 2
5 How to Use the Clearway 2 The Clearway 2 should only be used by trained carers/users of the device. Using the device without training may cause serious harm or injury to the patient. If you have not been trained on the device, contact the referring clinician or home care provider. -
Page 28: Circuits
Fit a bacterial filter close to patient mask, to minimise contamination of the Clearway 2 device and circuit. Circuits Standard MI-E Circuit with Filter Part Number: 008276 NIV and IPPB Circuit with Leak and Filter Part Number: 008277 Additional ‘Oxygen Coupler’ accessory available to entrain Oxygen to the... -
Page 29: Turning The Device On/Off
Alternatively press the standby button continuously for twenty seconds. On-Screen Symbols The Clearway 2 screen can contain the symbols and settings below. A summary of their meaning is included in the below table. Symbol Description Help button –... -
Page 30: General Functionality And Display
Exsufflation phase Recruitment Breaths General Functionality and Display The Clearway 2 is a touch screen device, and all settings and parameters are changed on-screen. Treatment is controlled only using the ‘hard’ buttons on the device (i.e. start/stop, manual switch, hand controller). - Page 31 Insufflation and Exsufflation pressure, respectively, set by the user. The Clearway 2 uses a Resistive Touchscreen enabling operation with users wearing gloves as part of their PPE. Resistive touch screen may not feel as responsive to users familiar with touchscreens and may require slightly more force to use than common devices such as smart phones.
- Page 32 The pin can be activated by pressing the desired setting twice to make the setting window stay visible on screen, even during treatment for live adjustments to the treatment settings. How to Use the Clearway 2 Clearway 2 User Manual DOC-001000 Ver 6...
-
Page 33: Main Menu
The Menu is accessed using the ‘Menu’ button at the bottom of the screen. The options available in the Menu are limited if the device is locked. Compliance Summary How to Use the Clearway 2 Clearway 2 User Manual DOC-001000 Ver 6... - Page 34 Synchrony Beep® The synchrony beep is intended to assist the patient and therapist/carer to synchronise their treatment, and hence improve effectiveness of the therapy. How to Use the Clearway 2 Clearway 2 User Manual DOC-001000 Ver 6...
- Page 35 See page Error! Bookmark not defined. for more information on Treat Repeat and Saved Profiles. Pressure Units The Pressure Units setting changes the measurement units for Pressure throughout the device, between cmH O and hPa. How to Use the Clearway 2 Clearway 2 User Manual DOC-001000 Ver 6...
- Page 36 The Reset Settings function allows the user to clear all user settings to defaults in the current mode, or all modes. Service Menu The service menu is accessed by a service code. Refer to Service Manual for Service Menu information. How to Use the Clearway 2 Clearway 2 User Manual DOC-001000 Ver 6...
-
Page 37: Treatment Settings
Options available in the Mode Select screen are limited if the device is locked. If the Clearway 2 device is unlocked, the prescribing physician or doctor is able to select any mode that is checked in the mode select screen. -
Page 38: Profiles
A cough treatment programmed in any of the MI-E modes, can be saved as a profile. Saving a treatment or TreatRepeat is only available when the Clearway 2 is unlocked. How to Use the Clearway 2 Clearway 2 User Manual DOC-001000 Ver 6... -
Page 39: Starting And Stopping Treatment
For Manual Treatment Options: • Using the front switch to manually activate therapy • Using the hand control to manually activate therapy How to Use the Clearway 2 Clearway 2 User Manual DOC-001000 Ver 6... -
Page 40: Recruitment Breaths
When set to OFF, Recruitment Breaths cannot be activated for treatment. Inspiratory pressure of the Recruitment Breaths are the same as the treatment insufflation pressure, set by the user How to Use the Clearway 2 Clearway 2 User Manual DOC-001000 Ver 6... - Page 41 Always ensure the patient has cleared any large airway secretions through expectorating, or suction, prior to starting the Recruitment Breaths, to prevent the secretions being pushed distally. How to Use the Clearway 2 Clearway 2 User Manual DOC-001000 Ver 6...
-
Page 42: Preview Treatment
It is not possible to Preview Treatment in the IPPB mode Active Treatment Display When the Clearway 2 is delivering treatment in an automatic Mi-E mode, you can press the Preview Treatment button to see a visual representation of the programmed treatment. -
Page 43: Entraining Oxygen
Always referrer to the oxygen provider’s instructions for safety. It is possible to use up to 15 litres per minute of low flow oxygen with the Clearway 2 if prescribed by your referring clinician. Only use the amount of oxygen as advised. - Page 44 The SpO reading will be displayed live on the main screen of the Clearway 2. The user will be notified with an alarm if the SpO sensor becomes disconnected. If the data collected from the sensor is corrupted or unreadable, the SpO reading will display a “?”...
-
Page 45: Accessing User Data
The internal battery is not designed to be user replaceable. Please contact technical support if a replacement is required. The Clearway 2 may be powered by an internal or an external battery. The Clearway 2 monitors the condition of the internal battery (if fitted) and automatically charges it as required when connected to the AC mains, and not in use. - Page 46 Battery Fault. Contact your provider Battery Under/Over Temperature <10°C or >45°C The battery LEDs on the front of the Clearway 2 will be solid if the battery is in use, and flash if the battery is charging. Internal Battery If available, an internal battery can be fitted inside the Clearway 2 (see Back Panel, section 3.4).
- Page 47 AC mains is the preferred power source. If the mains power supply is removed or fails, then Clearway 2 will switch to the External battery (if fitted) or internal battery (if fitted), in that order. Switching to battery power is indicated by an on-screen message, and the power source is indicated by an LED.
-
Page 48: Transferring Data
Transferring data Do not connect an SD card to the Clearway 2 during treatment. Downloading of data is prohibited by software during treatment. Compliance Data Patient compliance data may be downloaded to a PC via USB cable or SD card, by selecting ‘Compliance Summary’... -
Page 49: Alarms
6 Alarms Alarm Function The alarm function of the Clearway 2 consists of a yellow LED on the front panel, an audible alarm and a message box on the front LCD screen. Section 3.2, Front Panel, indicates the position of the LED. The visual alarm indicators... -
Page 50: Alarm Reset
An alarm condition will be automatically reset when the cause of the alarm has been corrected or the alarm condition no longer exists. If an alarm cannot be corrected, stop using the Clearway 2 and request technical support When an alarm has been acknowledged on screen, the alarm message will disappear unless the fault is reoccurring or still exists. - Page 51 Air inlet filters are blocked (e.g. placed on a bed or soft surface) Clearway 2 action Treatment will continue On Screen Message “Over Temperature! Switch device OFF. Leave for 30 minutes” Alarms Clearway 2 User Manual DOC-001000 Ver 6...
- Page 52 Incorrect breathing circuit is being used. Clearway 2 action Treatment continues. Alarm continues until condition resolved or treatment ends On Screen Message “Low leakage! Incorrect patient circuit for NIV - Ensure leak is fitted to the circuit” Alarms Clearway 2 User Manual DOC-001000 Ver 6...
- Page 53 Treatment will stop. On Screen Message “Machine Fault! Treatment has stopped! - Restart the Clearway 2 - Contact your supplier if fault persists” (Fault Code displayed is used to diagnose specific fault with the device) A Machine Fault can be caused by several different faults with the device.
- Page 54 Priority Medium Modes Possible Cause All power sources have been lost. Mains not connected, and batteries discharged Clearway 2 action Treatment not possible. Constant audio alarm and flashing LED On Screen Message Alarms Clearway 2 User Manual DOC-001000 Ver 6...
- Page 55 Treatment stops. The on-screen message must be acknowledged. Treatment cannot be started until message has been acknowledged On Screen Message “Pressure measurement fault! Treatment has stopped! - Restart the Clearway 2 - Contact your supplier if fault persists” Pressure Sensor Fault Item Description Definition...
- Page 56 Definition sensor has been disconnected Priority Modes Possible Cause sensor has been disconnected either at in- line connector or at Clearway 2 sensor has failed Clearway 2 action Treatment continues Message must be acknowledged to clear On Screen Message “SpO...
-
Page 57: Cleaning & Maintenance
Breas Clearway 2 Service Manual. • The Clearway 2 shall only be repaired or modified in accordance with the Breas, Clearway 2 Service Manual, technical bulletins, and any special service instructions, by service technicians that have been certified to do so after completing Breas Clearway 2 Technical Training. -
Page 58: Cleaning The Clearway 2
• Always be careful when cleaning the ensure that you do not damage any equipment. • Fluid must not be allowed to enter the Clearway 2. • Never apply any liquids directly on the Clearway 2 by spraying, splashing, or pouring. Use a moistened lint-free cloth when cleaning. -
Page 59: Changing The Air Inlet Filter
Replace the white filter at least every 4th week, or more frequently when used in high pollution or pollen-rich environments. Do not wash or reuse the disposable filter, this must always be replaced with a new filter. Cleaning & Maintenance Clearway 2 User Manual DOC-001000 Ver 6... -
Page 60: Change Of Patient
Change of Patient If the Clearway 2 is used in a clinic by several patients, use of a low resistant bacterial filter is essential between the air outlet and the patient tube to prevent patient cross-contamination. Follow the instructions in “Cleaning the Clearway 2” (page 57). -
Page 61: Essential And Regular Maintenance Control
Essential maintenance is required for the Clearway 2 after 5 years of service (please see Clearway 2 Service Manual for further details). Breas advise annual checks to the Clearway 2 to ensure electrical safety as well as flow and pressure calibration. The Service Manual outlines the test instructions for Electrical Device Safety Testing which should be carried out by trained service personnel. -
Page 62: Storage
Storage Store the Clearway 2 device in a dark room, where the temperature range is within -20°C to +60°C (-4°F to +°140F). For instructions on how to charge the batteries after long time storage, see “Using Batteries” (page 45). • The Clearway 2 must not be stored in a warm place, such as direct sunlight, close to a radiator or in a vehicle in sunlight. -
Page 63: Faq's & Troubleshooting
See section 7.7 for further transport information. 2. What is the service life of the Clearway 2? The service life of the Clearway 2 is 7 years if it is continuously regularly serviced and maintained. All other Breas parts and accessories included or used with the Clearway 2 have a 7-year service life. - Page 64 6. What interfaces can you use for IPPB? The suggested interface for IPPB mode is a mouthpiece. If the patient cannot seal around the mouthpiece an oronasal mask can be used. FAQ’s & Troubleshooting Clearway 2 User Manual DOC-001000 Ver 6...
-
Page 65: Troubleshooting
Send device for service External battery will not charge a. Check mains power connection and the standby light is on b. Check battery connector is inserted correctly to the Clearway 2 c. Replace External battery d. Send device for service FAQ’s &... -
Page 66: Technical Specifications
9 Technical Specifications System Description Technical Specifications Clearway 2 User Manual DOC-001000 Ver 6... -
Page 67: Data Parameters
Resolution 0.1 second Tolerance ± 0.25 seconds 0 – 20 Hz Oscillation Frequency Resolution 1 Hz Tolerance ± 1 Hz 0 - 10 cmH Oscillation amplitude Resolution 1 cmH Tolerance ± 1 cmH Technical Specifications Clearway 2 User Manual DOC-001000 Ver 6... -
Page 68: Electrical Information
50 to 60 Hz, max 260 W Type of protection against Class II Electric Shock Degree of protection Type BF Applied Part against Electric Shock Ingress Protection Rating Ingress Protection rating: IP22 Mode of Operation Continuous Technical Specifications Clearway 2 User Manual DOC-001000 Ver 6... -
Page 69: Environmental Operating Conditions
IP22 dripping water less than 15 degrees from vertical. The ingress protection has been tested by water drips equivalent to 3mm rain/minute for 10 minutes (2.5 minutes for each tilting direction) Technical Specifications Clearway 2 User Manual DOC-001000 Ver 6... -
Page 70: Dimensions
CLEARWAY 2 DIMENSIONS SPECIFICATIONS W x H x D 285 x 285 x 195 mm Weight 3.75 kg (without internal battery) 22 mm male, 15 mm female conical Patient air outlet standard connector Technical Specifications Clearway 2 User Manual DOC-001000 Ver 6... -
Page 71: Emissions And Immunity Declaration
Emissions and Immunity Declaration Note: The ‘Essential Performance’ of the Clearway 2 is defined as follows: Clearway 2 Essential Performance The Clearway 2 will deliver therapeutic treatment via the patient-connection port (outlet) at the set pressure (+/- 10% of full scale). -
Page 72: Compliance Of Standards
Compliance of Standards Guidance and Manufacturer’s Declaration Safety Standards Safety Standard IEC EN 60601-1 IEC EN 60601-1-1 IEC EN 60601-1-2 IEC EN 60601-1-6 IEC EN 60601-1-8 IEC EN 60601-1-11 IEC ISO 80601-2-61 Technical Specifications Clearway 2 User Manual DOC-001000 Ver 6... - Page 73 0% UT, 1 cycle; environment. If the user of variations on 70% UT, 25/30 cycles the Clearway 2 requires power supply (50/60 Hz); continued operation during input lines 0% UT, 250/300 cycles power mains interruptions, it IEC 61000-4-11 (50/60 Hz);...
- Page 74 Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the Clearway 2, including cables specified by the manufacturer. Otherwise, degradation of the performance of this equipment could result.
- Page 75 Compliance Electromagnetic Environment - Guidance RF emissions Group 1 The Clearway 2 uses RF energy only for CISPR 11 its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment.
- Page 76 Recommended separation distances between portable and mobile RF communications equipment and the Clearway 2 The Clearway 2 is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the Clearway 2 can help prevent electromagnetic interference by maintaining a...
-
Page 77: Clearway 2 In Other Countries
Recommended separation distances between external power conductors and the Clearway 2 Rated Maximum Current in Separation Distance (M) Conductor (A) 50-60 Hz d= I/2πH= I/188 0.005 0.05 0.16 For conductors rated at a maximum current not listed above, the recommended separation distance d in metres (m) can be estimated using the equation d=I/2πH, where I is the maximum current rating of the conductor... -
Page 78: Accessories
10 Accessories Only use accessories recommended by Breas Medical Ltd. Breas Medical cannot guarantee the performance and safety for the use of other accessories with the Clearway 2. Accessory equipment connected to the analogue and digital interfaces must be certified according to the respective IEC standards (e.g. IEC 60950 for data processing equipment and IEC 60601-1 for medical equipment). -
Page 79: Optional Accessories
Sensor Paediatric 006590 Memory Card 006705 USB Cable 004886 Internal Battery 008424 Use with other manufacturer’s accessories or non-approved Breas accessories supplied or recommended by Breas may cause the device to malfunction. Accessories Clearway 2 User Manual DOC-001000 Ver 6... -
Page 80: Patient Settings
Patient Settings This page can be copied and used for noting the patient’s settings. Patient Settings – Breas Clearway 2 Patient ………………………………………………………………… Date ………………………………………………………………… Clinic ………………………………………………………………… Set by ………………………………………………………………… Patient Circuit ………………………………………………………… Mode ………………………………………………………………… INS Pressure ………… Ins Trig ………….…… EXS Pressure……………... -
Page 81: Glossary Of Terms
(NIV) times TreatRepeat® is the ability to save a manual treatment TreatRepeat® performed into a pre-set programme, therefore reducing time for titration as well as enhancing patient synchrony Glossary of Terms Clearway 2 User Manual DOC-001000 Ver 6... -
Page 82: Index
Note Symbol................................5 On-Screen Symbols .............................. 29 Oxygen ................................18, 43 Oxygen Connector ..............................79 Package Contents ..............................78 Preparing the Clearway 2 for Use ......................... 25 Pressure Units............................... 35 Product Description ............................... 20 Index Clearway 2 User Manual DOC-001000 Ver 6... - Page 83 Synchrony Beep ..............................34 Treatment Settings ..............................37 Troubleshooting ..............................65 Undesirable Side Effects ............................12 USB ..................................48 User Preferences ..............................34 Warning Symbols ..............................5 Warnings ................................9 Weight ................................... 70 Index Clearway 2 User Manual DOC-001000 Ver 6...
- Page 84 Företagsvägen 1 SE-435 33, Mölnlycke Sweden Phone: +46 31 868800 Email: breas@breas.com Breas Medical Ltd · UNIT A2, The Bridge Business Centre, Timothy’s Bridge Road Stratford-upon-Avon, England, CV37 9HW Phone: + 44 (0) 1789 293 460 E-mail: uk@breas.com www.breas.com Company registered in England and Wales...















Need help?
Do you have a question about the Clearway 2 and is the answer not in the manual?
Questions and answers