Advertisement
Quick Links
Advertisement

Subscribe to Our Youtube Channel
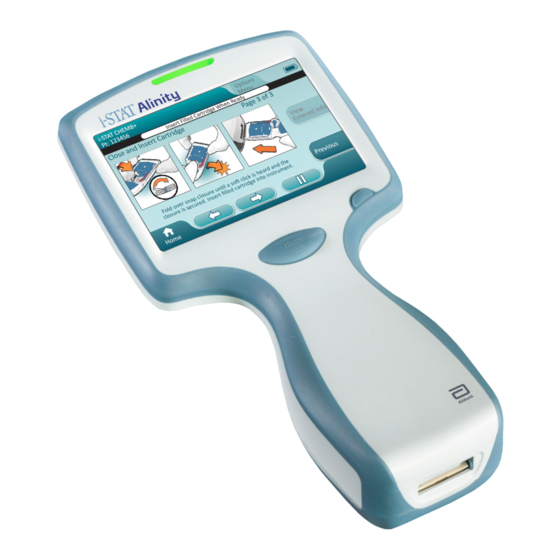
Summary of Contents for Abbott i-STAT Alinity
- Page 1 Alinity Quick Reference Guide...
- Page 2 This page left intentionally blank. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 3 HOW TO PERFORM SOFTWARE UPDATE APPENDIX 1: SYMBOLS, TERMINOLOGY AND TEST ABBREVIATIONS INTENDED USE: The i-STAT Alinity instrument is intended for use in the in vitro quantification of various analytes in whole blood or plasma in point of care or clinical laboratory settings. SCOPE: The Quick Reference Guide contains information that describes several functional pathways of the i-STAT Alinity instrument.
- Page 4 This page left intentionally blank. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 5 CHARGE CONTINUE TO STEP 4. An alert displays when the battery level approaches the level at which testing is disabled. CHARGE BATTERY IF NEEDED OR MOVE TO STEP 4 i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 6 SECTION 1 INSTRUMENT SETUP (New Instruments) CONT. If instrument displays Set Region Code screen, proceed to STEP If not, proceed to STEP LOCATE REGION BAR CODE i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 7 4. Once the instrument powers on, the Region Code Barcode alert should no longer be displayed. Proceed to STEP If Alert screen displays again, repeat STEP If the Alert screen displays again, contact your Abbott representative. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev.
- Page 8 SECTION 1 INSTRUMENT SETUP (New Instruments) CONT. FINISH SETTING UP THE INSTRUMENT Power instrument on, follow sequence and on-screen instructions. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 9 SECTION 1 INSTRUMENT SETUP (New Instruments) CONT. FINISH SETTING UP THE INSTRUMENT CONT. Set Language Set Clock i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 10 Set Units NOTE: Not all cartridges are available in all regions. Check with your local representative for availability in specific markets. Set Date Format INSTRUMENT SETUP IS COMPLETE i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 11 ALINITY ELECTRONIC SIMULATOR: Provides an independent check on the instrument’s thermal controls and success of software updates. i-STAT ALINITY PRINTER: Used to print records stored in the instrument. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev.
- Page 12 • use the instrument in environmental conditions that exceed the operating temperature and humidity specifications. • make any unauthorized repairs or modifications to this product as this may cause personal injury or damage to the unit. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 13 To disable automatic date/time synchronization, run the Set Clock flow and uncheck the box Synchronize Clock with Data Manager. Then press Set Date/Time manually and set the correct date/time. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 14 • charge a new rechargeable battery pack for 4 hours before initial use. A fully discharged battery will be 100% charged and ready for use after 4 hours. • use only a rechargeable battery pack purchased from Abbott Point of Care. • use only the accessories and consumables specified or supplied for this system by Abbott Point of Care.
- Page 15 • In the event of a battery leaking, do not allow any leakage to come into contact with the skin or eyes. If contact has been made, wash the affected area with copious amounts of water and seek medical advice. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M...
- Page 16 • always use the Base Station for charging. Refer to the rechargeable battery Getting Started Guide on instructions for proper charging. • connect only Abbott Point of Care provided printers to the Base Station printer port. • check with authorities for local, state, and/or national requirements for disposal.
- Page 17 Base Station. • Protection provided by this equipment may be impaired if used in a manner not specified by Abbott Point of Care. • The instrument and its peripherals are not listed by any authority with respect to suitability for use in oxygen-enriched atmospheres.
- Page 18 Alinity printer. • use only a rechargeable battery pack purchased from Abbott Point of Care. • use only the power adapter and supply provided with the i-STAT Alinity printer kit. • use an i-STAT Alinity printer when attempting to print from an i-STAT Alinity instrument.
- Page 19 • Fluorescent light sources can cause interference with communications sent to the i-STAT Alinity printer. When light from a fluorescent source of sufficient proximity or brightness has a direct path into the printer’s infrared radiation (IR) window, the printer might fail to respond when records are sent for printing over a serial (wired) connection to a Base Station.
- Page 20 The IR port sends information from the instrument to the portable printer. BATTERY: Rechargeable battery is the sole power source for the instrument. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 21 SCREEN COMPONENTS AND THEIR MEANINGS After the power button is pushed and the instrument starts the power- up sequence, the LED light will turn green, and i-STAT Alinity will appear briefly on the display screen. During the power-up sequence, the i-STAT Alinity instrument performs a series of self-checks.
- Page 22 ® Buttons provide access to pathways ¯ Perform Patient Test ¯ More Options FOOTER Area Contains: ® Home Button * See Page 21 for screen icons and their meanings. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 23 Indicates status of alert. Instrument is locked until requirement is satisfied Instrument warning ALERTS INDICATOR: Displays number of alerts ALERT TITLE ALERT DESCRIPTION: Displays cause and resolution i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 24 SIDE ACTION TABS: ® Provide access to area or action indicated ALERT BUTTON: ® Provides access to Alerts description ACTION BUTTONS: ® Provide options for screen navigation i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 25 Bolt indicates active- Connection ly charging Warning Wireless Bolt indicates active- Disabled ly charging Instrument Wireless locked Connecting Wireless Information Allowed Instructional Icons Low Battery Mandatory i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 26 Refrigerated storage temperature indicator: 2-8°C (35-46°F) Indicates shelf life when stored at room temperature Refrigerated storage expiration date Cartridge LOT number Location to record room temperature expiration date i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 27 2D barcode for manufacturing quality control; not scannable Cartridge LOT number Cartridge pouch barcode Refrigerated storage expiration date Indicates shelf life when stored at room temperature Room temperature storage range i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 28 Analytes – measured and calculated, if applicable 2D barcode for manufacturing quality control; not scannable Cartridge LOT number Cartridge portion pack barcode Refrigerated storage expiration date Refrigerated temperature storage range i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 29 HOW TO PERFORM QUALITY TESTING MANUFACTURER’S QUALITY SYSTEM INSTRUCTIONS (MQSI) The list below defines the i-STAT Alinity System MQSI components. Check New or Replacement Instruments with the Electronic Simulator Use the Electronic Simulator to verify operation of a new or replacement instrument before use.
- Page 30 HOW TO PERFORM QUALITY TESTING (CONT.) ELECTRONIC SIMULATOR The i-STAT Alinity Electronic Simulator is a quality control device used to evaluate the i-STAT Alinity instrument’s ability to read the electronic signal from a cartridge. The test cycle for the Electronic Simulator is approximately 60 seconds.
- Page 31 • place the Electronic Simulator in an oxygen-enriched atmosphere. • make any unauthorized repairs or modifications to this product. • use the Electronic Simulator with any instrument other than the i-STAT Alinity. • touch the area under the cap. NOTE: •...
- Page 32 Use care when handling the Electronic Simulator. Avoid touching the sensor area. Replace cap after use. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 33 ¯ On screen graphics and text are provided to assist the user. NOTE: Not all cartridges are available in all regions. Check with your local representative for availability in specific markets. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 34 Scheduled QC is only available when set by the System Administrator. The next step in the pathway is Scan or Enter OPERATOR ID Quality Control Test Scan or Enter OPERATOR ID Next Home i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 35 Quality Control Test Scan CARTRIDGE POUCH Barcode Next View Entered Info Previous Home Scan CARTRIDGE POUCH Barcode. Manual entry is not an option. Scanning is required. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 36 Once the cartridge is inserted, Contacting Cartridge will display followed by the countdown bar. This allows the user to estimate the time to results. Alerts such as Cartridge Locked and Instrument Must Remain Level are also displayed. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 37 *** – (Starouts) Instrument is unable to determine a result. A sample may also yield results that are preceded by a greater than (>) or less than (<) symbol. These results are outside the instrument’s measurement range. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M...
- Page 38 Entered Info 2.6 - 3.2 3.0 - 4.6 0.73 - 0.89 Cl, mmol/L AnGap, mmol/L Print 68 - 77 TCO2, mmol/L Transmit 8 - 26 Page Home i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 39 • The instrument and its peripherals are not listed by any authority with respect to suitability for use in oxygen-enriched atmospheres. • Follow manufacturer’s recommendations for handling and storing samples drawn into lithium or balanced heparin. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 40 Standard precautions should be used when handling materials that may contain transmissible infectious agents. Some regions have an alternate patient test flow. Always follow prompts on the screen. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 41 For alpha, touch the Abc button. After entering the information, touch Enter and the instrument will advance to the next step in the pathway. Enter OPERATOR ID and then touch Enter Enter @#& Close i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 42 This information cannot be entered manually. Pa�ent Test Pt: 123456 Scan (CARTRIDGE POUCH) Barcode Next View Entered Info Previous Home If an Invalid Cartridge Type window is displayed, contact the System Administrator. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 43 The action buttons at the bottom of the screen allow forward, backward and pause functionality. For experienced users, the help screens may be bypassed by inserting a filled cartridge. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 44 CHEM8+ Pt: 123456 Tes�ng - Instrument Must Remain Level Contac�ng Cartridge View Entered Info Cartridge locked in instrument. Do not a�empt to remove the cartridge. Home i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 45 (<) symbol. These results are outside the instrument’s measurement range. In order to determine the exact numeric result, the sample must be analyzed by a different method. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 46 Na, mmol/L BUN, mg/dL Glu, mg/dL Op�ons Menu K, mmol/L Crea, mg/dL iCa, mmol/L View 0.94 Entered Info Cl, mmol/L AnGap, mmol/L Print TCO2, mmol/L Page Home i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 47 View 1.33 Entered Info 0.7 - 1.5 AnGap, mmol/L Print Transmit Page Symbol indicates instrument is connected to the network To initiate transmission, touch Transmit i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 48 (critical) range. ¯ White arrow in page button - indicates all results on second page are within the reference range. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M...
- Page 49 Alinity Instrument, Base Station, Printer and Electronic Simulator It is recommended that the i-STAT Alinity, base station, printer and electronic simulator be cleaned periodically or whenever visibly soiled. Standard precautions should be taken whenever working with blood or blood products.
- Page 50 SENSITIVE AREAS Avoid forcing liquid into these areas: i-STAT Alinity Instrument Cartridge Port 10-Pin Connector under the camera Gold contacts (2) on the outside of the battery i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 51 Avoid forcing liquid into these areas: Electronic Simulator Area between protective cap retaining ring and white sensor area Base Station 10-Pin Connector Gold contact pins (2) USB Port i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 52 SECTION 8 CLEANING AND DISINFECTING (CONT.) • Due to the portability of the i-STAT Alinity Instrument, it may be subject to splatter or splash of bodily fluids when used in proximity of patients. Failure to wear clean gloves will result in contamination of the instrument.
- Page 53 SECTION 9 TROUBLESHOOTING AND SUPPORT TROUBLESHOOTING The i-STAT Alinity is programmed to perform quality checks throughout the testing cycle. The instrument has several methods of notifying operators of failed quality checks. 1. Quality Check Failures - Are displayed when the instrument identifies a problem while running a...
- Page 54 Once you have registered for access, login and navigate to SUPPORT > i-STAT 1 and i-STAT Alinity SUPPORT > CHOOSE YOUR PRODUCT > i-STAT ALINITY RESOURCES LOGIN and select from the following: • Value Assignment Sheets •...
- Page 55 SECTION 10 HOW TO PERFORM SOFTWARE UPDATE Software updates to the i-STAT Alinity instrument are delivered twice a year. Each software update contains two elements in a single package: CLEW software and application software. Note: Best practice is to enable use of an Operator List to protect the Update Software flows from being executed by unauthorized personnel.
- Page 56 OSi Software Installation Results: The instrument automatically restarts upon successful installation! Refer to onscreen details for cause and repeat procedure. If failure persists, contact your local Abbott representative. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 57 Date of manufacture. Manufacturer. In vitro diagnostic medical device. For prescription use only. Authorized Representative for Regulatory Affairs in the European Community. Importer in the European Community. Control. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 58 400 College Road East be used in a near-patient setting by a health worker, professional or Princeton, NJ 08540 (609) 454-9304 trainee. christopher.fetters@abbott.com Rev: 01 Feb 2010 i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 59 APPENDIX 1 SYMBOLS (CONT.) SYMBOL DEFINITION Information Instrument Locked Low Battery Pass Warning Fail i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 60 Quality Check Failure Quick Reference Guide R-VAS Rilibak Value Assignment Sheet ReVAS Rilibak Electronic Value Assignment Sheet Software Update Traumatic Brain Injury Universal Serial Bus Value Assignment Sheet i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 61 Base excess (b for blood, ecf for extracellular fluid) AnGap Anion Gap Oxygen saturation eGFR Estimated Glomerular Filtration Rate Black/African-American Estimated Glomerular eGFR-a Filtration Rate GFAP Glial Fibrillary Acidic Protein UCH-L1 Ubiquitin Carboxy-terminal Hydrolase L1 i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 62 IS FOUND TO HAVE FAILED OF ITS ESSENTIAL PURPOSE. SOME STATES DO NOT ALLOW THE LIMITATION AND/OR EXCLUSION OF LIABILITY FOR INCIDENTAL OR CONSEQUENTIAL DAMAGES, SO THE ABOVE LIMITATION OR EXCLUSION MAY NOT APPLY TO YOU. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 63 This page left intentionally blank. i-STAT Alinity — Quick Reference Guide Art: 731848-01 Rev. M Rev. Date: 25-Jul-2022...
- Page 64 In Vitro Diagnostic Use. Printed in the USA. For information related to Article 33 of the EU REACH regulation (EC No. 1907/2006), please refer to PMIS.abbott.com. If you have issues logging into the website,contact Abbott at: abbott.REACH.abbott.com. Emergo Europe Abbott Point of Care Inc.











Need help?
Do you have a question about the i-STAT Alinity and is the answer not in the manual?
Questions and answers