Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Integra Codman VPV System
- Page 1 Codman ® VPV® System...
- Page 2 This page is intentionally left blank.
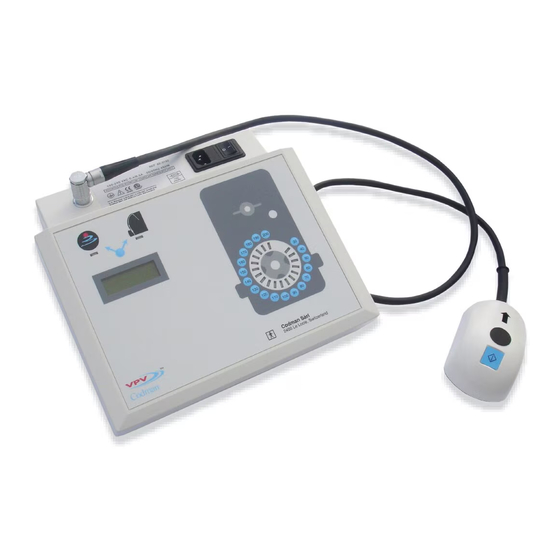
- Page 3 A. Start button B. Direction-of-flow arrow C. Cord D. Center rod E. Feet (4 total) A. Program Unit B. Transmitter Unit Adjusting a packaged valve A. Liquid crystal display (LCD) screen F. Setting selection keys and their corresponding LEDs A. Programming valve within sterile blister package B.
- Page 4 1. Cylindrical Valve 2. Micro Valve Adjusting an implanted valve (using implanted valve mode) A. Patient’s scalp B. Inlet portion of valve C. Ultrasound gel on center rod of transmitter D. Direction of flow ® 3. Micro Valve with RICKHAM Reservoir ®...
- Page 5 Correlation of positions of the cam position (pressure) indicator to the desired settings Pressure indicator, as seen on packaged valve (magnified) A. Pressure indicator, shown at “70” setting B. Cam C. Flat spring D. Direction of flow...
-
Page 6: Table Of Contents
EN – ENGLISH Table of Contents Indications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 Contraindications . -
Page 7: Indications
Implanted Product Description Valve Icon The CODMAN VPV System (catalog no. 823192R) comprises the program unit, the transmitter, a 3m long power cord, and ultrasound gel in a carrying case (see Figures 1 through 3). The Acoustic Monitoring Feature Function and Intended Application When the implanted valve mode is selected, a sensor contained within the transmitter detects valve vibration as the setting of the valve is changed. -
Page 8: Adjusting The Valve
EN – ENGLISH Do not use the program unit in a magnetic resonance imaging (MRI) suite. The LCD panel displays the following message: Electromagnetic interference and other types of interference generated by other equipment or medical devices might IMPLANTED VALVE affect the operation of the system. -
Page 9: Valve Adjustment: Implanted Valve Mode
EN – ENGLISH When the adjustment is complete (approximately 3 seconds), the program unit emits one long beep and the Place the transmitter on the scalp so the center rod is in the center of the ultrasound gel (directly over the hard display changes to: inlet portion of the valve) and the transmitter’s feet contact the patient’s scalp. -
Page 10: Valve Adjustment: Packaged Valve Mode With An Implanted Valve
EN – ENGLISH Valve Adjustment: Packaged Valve Mode With an Implanted Valve To adjust a newly implanted valve, use a sterile drape to protect the patient from contact with the transmitter. Follow the instructions below. Note: Select the Packaged Valve mode when a drape is used because the Implanted Valve mode will likely result in a “REPEAT ADJUSTMENT”... -
Page 11: Troubleshooting
EN – ENGLISH Troubleshooting LCD displays the following message Cause Solution The signal received was not sufficient to confirm the expected movement of Press any key; repeat the adjustment sequence, including palpating the valve, the valve. Possible causes include: verifying the amount of ultrasound gel, and positioning the transmitter. Incorrect placement of the transmitter REPEAT ADJUSTMENT If the message continues to appear, an x-ray must be taken to confirm the... - Page 12 EN – ENGLISH Troubleshooting LCD displays the following message Cause Solution Transmitter is disconnected from the program unit Reconnect the transmitter to the program unit Continued occurrence of this message might indicate a technical problem; return for service. TRANSMITTER HEAD DISCONNECTED Technical problem with the transmitter’s acoustic monitoring feature Replace the transmitter.
-
Page 13: Specifications
Disconnect the program unit from the power supply. FROM USE OF THESE PRODUCTS. INTEGRA NEITHER ASSUMES NOR AUTHORIZES ANY PERSON TO ASSUME ANY OTHER OR The program unit’s fuse holder is located adjacent to the power switch on the rear of the top panel. Use a small ADDITIONAL LIABILITY OR RESPONSIBILITY IN CONNECTION WITH THESE PRODUCTS. -
Page 14: Appendix B: The Effect Of Ambient Temperature On Valve Adjustment
EN – ENGLISH Appendix B: The Effect of Ambient Temperature on Valve Adjustment To select one of the languages displayed, press the appropriate key. To view the next three language choices, press the “200” key until the desired language is displayed. The available languages are: Ambient temperature affects the number of adjustment cycles that can be completed before the transmitter reaches the •... -
Page 15: Appendix E: Clinical Study Summary
EN – ENGLISH Table 2 – Guidance and Manufacturer’s Declaration – Immunity Press the “30” key to set the next adjustment cycle for an inverted valve, or press “40” to exit. When you press “30,” the display changes to: All ME Equipment and ME Systems Guidance and Manufacturer’s Declaration - Immunity ADJUST INVERTED The VPV Programmer is intended for use in the electromagnetic environment specified below. - Page 16 EN – ENGLISH Table 3 – Guidance and Manufacturer’s Declaration – Immunity Table 4 – Recommended Separation Distances between portable and mobile RF Communications ME Equipment and ME Systems that are NOT Life-supporting equipment and the VPV Programmer ME Equipment and ME Systems that are NOT Life-supporting Guidance and Manufacturer’s Declaration –...
- Page 17 EN – ENGLISH This page is intentionally left blank.
- Page 18 EN – ENGLISH This page is intentionally left blank.
- Page 19 Caution: Federal (US) law restricts this device to sale by or on the order of a physician. Made in Caution Waste Electrical and Electronic Equipment Quantity Consult Instructions for Use Follow Instructions for Use Type BF – Equipment with an electrical path to the patient, not including direct cardiac application Packaged Valve Mode Fuse Alternating Current...
- Page 20 CODMAN, VPV, RICKHAM, SIPHONGUARD, Integra and the Integra logo are registered trademarks of Integra LifeSciences Corporation or its subsidiaries in the United States and/or other countries. HAKIM is a registered trademark of Hakim USA, LLC and is used under license. ©2020 Integra LifeSciences Corporation. All rights reserved. LCN 208525-001 Rev. D 01/20 1088089-4...














Need help?
Do you have a question about the Codman VPV System and is the answer not in the manual?
Questions and answers