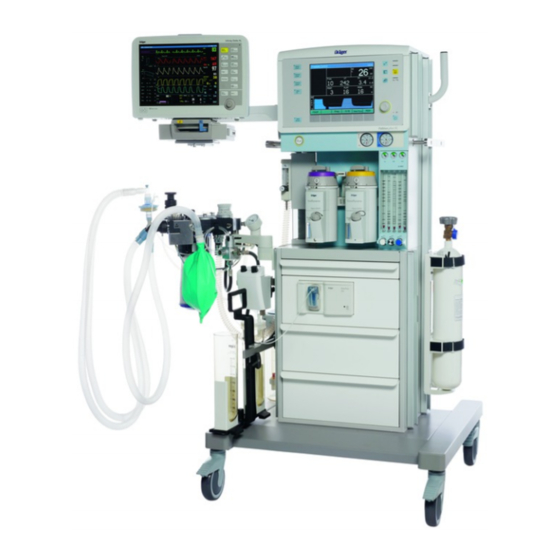
Dräger Fabius plus Supplement Manual
Anesthesia workstation software 3.n
Hide thumbs
Also See for Fabius plus:
- Instructions for use manual (232 pages) ,
- Supplement to instructions for use (142 pages) ,
- Supplement manual (110 pages)
Summary of Contents for Dräger Fabius plus
- Page 1 Supplement Fabius plus Anesthesia workstation WARNING Software 3.n To properly use this medical device, read and comply with the instructions for use and this supplement.
- Page 2 Trademark owner ® Ecolab Actichlor ® Brulin BruTab 6S ® Buraton ® Schülke & Mayr Mikrozid ® Perform ® Antiseptica Descogen ® Bode Chemie Dismozon ® Medentech Klorsept ® Ecolab USA Oxycide ® DuPont Virkon Supplement Fabius plus SW 3.n...
- Page 3 This device can be affected by other electrical gloves. devices. – Observe the requirements for the electromagnetic environment. Observe the following section: "Electromagnetic environment" (page 46). Supplement Fabius plus SW 3.n...
- Page 4 – Maintain a distance of at least 0.3 m (1.0 ft) between this device and wireless communication devices, to ensure that the essential performance of this device is fulfilled. – Maintain an adequate distance between this device and other medical electrical equipment. Supplement Fabius plus SW 3.n...
-
Page 5: Product-Specific Safety Information
– Use only the handles provided to push or pull the device. The following safety information has been deleted: WARNING Risk of device failure The device can fail if the power supply is interrupted. Always connect the device on an uninterruptible power supply. Supplement Fabius plus SW 3.n... - Page 6 The following safety information has been added: Overview Compact breathing system COSY (top view) The following designation has changed: N Connection for sample gas return line Supplement Fabius plus SW 3.n...
- Page 7 Overview The views of the power supply unit for the COSY heater have changed: Power supply unit for COSY heating (front view) A LED indicator for COSY heating B On/Off switch C Fuse Supplement Fabius plus SW 3.n...
- Page 8 Overview Power supply unit for COSY heating (rear view) A Power inlet B LED indicator for power supply C Cable clamp Supplement Fabius plus SW 3.n...
-
Page 9: Interface Panel
C Connection for APL hose D Socket for O sensor E Socket for airway pressure sensor F Socket for flow sensor G Power inlet with power fuses H On/Off switch Fuse for internal battery J Potential equalization pin Supplement Fabius plus SW 3.n... - Page 10 – Make sure that there is sufficient clear space. – Remove any devices mounted on attached support arms or on the top of the device. – Fold in the support arms. – Clear the writing tray and push it in completely. Supplement Fabius plus SW 3.n...
- Page 11 – Make sure that there is sufficient clear space. – Remove any devices mounted on attached support arms or on the top of the device. – Fold in the support arms. – Clear the writing tray and push it in completely. Supplement Fabius plus SW 3.n...
- Page 12 B Long fresh-gas hose (on Fabius) C APL bypass hose for the ventilator lock D Short fresh-gas hose (on compact breathing system) E Compact breathing system F Sample line G Non-rebreathing system (e.g., Bain) Supplement Fabius plus SW 3.n...
- Page 13 Keep free of oil and grease DC voltage AC voltage Fragile, handle with care Keep dry Connection for sample gas gas return return line The fiollowing symbol has been deleted: Caution! Risk of electric shock. Do not remove cover. Supplement Fabius plus SW 3.n...
-
Page 14: Product Labels
COSY position. For further information, see "Using the external fresh-gas outlet with an additional switch (optional)" in the "Operation" chapter in the instruc- tions for use. Supplement Fabius plus SW 3.n... -
Page 15: Assembly And Preparation
If the battery has not been charged for 6 months, check its functional integrity. To do this, charge the battery for 16 hours and ventilate a test lung for at least 45 minutes in battery operation. Supplement Fabius plus SW 3.n... - Page 16 >700 mL 301 to 700 mL 50 to 300 mL <50 mL Breathing bag 0.5 L Breathing circuit Adults Pediatric Neonates (or pediatric) Filter Filter, HMEF, or HME Use filters with low resistance and compliance. Supplement Fabius plus SW 3.n...
- Page 17 O sensor housing. 5 Screw on the screw cap (C) tightly by hand. 6 Insert the O sensor (A) back in the inspiratory valve. Supplement Fabius plus SW 3.n...
- Page 18 A and B. 2 Connect the short APL bypass hose to connectors E and F. 3 Connect the PEEP/P hose to connectors C and D. NOTE The APL bypass hose is thicker than the PEEP/P hose. Supplement Fabius plus SW 3.n...
-
Page 19: Operation
When the switch of the external fresh-gas outlet is set to operation with a non-rebreathing system ], no alarm occurs at the start of the ManSpont ventilation mode. Check that the switch of the external fresh-gas outlet has been set to COSY. Supplement Fabius plus SW 3.n... - Page 20 Oxygen and anesthetic gas are no longer delivered. Due to the patient’s rebreathing, the inspiratory concentration of anesthetic gases and oxygen in the breathing gas falls. Monitor the gas concentrations carefully and use intravenous anesthetic agents if necessary. Supplement Fabius plus SW 3.n...
- Page 21 3 If necessary, connect the non-rebreathing system scavenging hose to the second connection (D) of the anesthetic gas receiving system. Observe the instructions for use of the non- rebreathing system and the anesthetic gas receiving system. Supplement Fabius plus SW 3.n...
- Page 22 – VENTILATOR FAIL !!! – CHECK APL VALVE !!! Ending operation 1 Close all flow control valves on the device. Before using a controlled ventilation mode (Volume Control, Pressure Control, Pressure Support, SIMV/PS) or the ManSpont ventilation mode: Supplement Fabius plus SW 3.n...
-
Page 23: Ending Operation
Y-piece or filter is plugged onto the circuit plug and if a reprocessed component is attached later in the process (e.g., during a leakage test), the new component may become contaminated. Only plug reprocessed components onto the circuit plug. Supplement Fabius plus SW 3.n... - Page 24 – Do not pull the medical device over hoses, cables, or other obstacles lying on the floor. – Do not activate the brake while the medical device is being moved. – Use only the handles provided to push or pull the device. Supplement Fabius plus SW 3.n...
-
Page 25: Leakage Test
Contact DrägerService or the authorized local service partner Results of the compliance test The value for the system compliance has changed: System compliance Displayed result [mL/cmH [mL/cmH ≤ Measured value and PASSED Supplement Fabius plus SW 3.n... - Page 26 3 Select new unit and confirm. The window is closed. 1 Select menu item Pressure Unit (G) and confirm. The screen with the password query opens. Standby Set-up 2 Enter the password (H) and confirm. Supplement Fabius plus SW 3.n...
-
Page 27: Troubleshooting
3 Set the APL valve to the Man position. 4 Set the APL valve to the desired pressure. 5 Fill the breathing bag, if necessary with the aid of the O flush key. 6 Manually ventilate the patient. Supplement Fabius plus SW 3.n... - Page 28 Check that the O sensor capsule has been cor- rectly inserted into the O sensor housing. Connect the O sensor correctly and recalibrate. Supplement Fabius plus SW 3.n...
-
Page 29: Alarm - Cause - Remedy
Leakage in the breathing sys- Check breathing system. tem. Hose not connected to the breathing system. Incorrect setting of the switch Check the setting of the on the external fresh-gas out- switch on the external let. fresh-gas outlet. Supplement Fabius plus SW 3.n... - Page 30 Check APL valve setting. Switch of the external fresh- Set the switch of the exter- gas outlet in the wrong posi- nal fresh-gas outlet to the COSY position. tion ( ), instead of the COSY position. Supplement Fabius plus SW 3.n...
- Page 31 Before removing the compact breathing system, the following hoses and cables must be removed: – Flow sensor cable – O sensor cable and O sensor – Pressure measurement hose – APL bypass hose – PEEP/PMAX hose Supplement Fabius plus SW 3.n...
-
Page 32: Safety Information
– Before inserting the flow sensor, check for visible damage and soiling such as Check the products for signs of wear and replace residual mucus, medication aerosols, and them if necessary. particles. – Replace flow sensors when damaged, soiled, or not particle-free. Supplement Fabius plus SW 3.n... -
Page 33: Classification Of Medical Devices
Semi-critical Components that carry breathing gas or come into contact with mucous membranes or pathologi- cally altered skin Critical Components that penetrate skin or mucous membranes or come into contact with blood Supplement Fabius plus SW 3.n... - Page 34 – Flow sensor – Gas cylinder valves Critical – Transfer hose of the anesthetic gas The device does not contain any components receiving system classified as critical. – Cables and hoses that lie on floor – Brake Supplement Fabius plus SW 3.n...
-
Page 35: Reprocessing List
APL valve Yes (exterior) Inspiratory valve, expiratory valve Bag elbow Rigid arm for breathing bag (optional) Flexible arm for breathing bag (optional) Absorber con- tainer and absorber insert Clic adapter (option) Ventilator lid Ventilator mem- brane Supplement Fabius plus SW 3.n... - Page 36 "Validated reprocessing procedures". The manufacturers of the surface disinfectants have verified at least the following spectra of activity: – Bactericidal – Yeasticidal – Virucidal or virucidal against enveloped viruses Supplement Fabius plus SW 3.n...
- Page 37 1) Discontinued by the manufacturer 2) Virucidal against enveloped viruses Dräger states that oxygen-releasing agents and chlorine-releasing agents may cause color change in some materials. Color change does not indicate that the product is not functioning correctly. Supplement Fabius plus SW 3.n...
- Page 38 Make sure that 6 Allow the product to dry completely. the solution reaches all surfaces and interior spaces. 3 Rinse the product with water (at least drinking- water quality) until residual cleaning agent is no longer discernible. Supplement Fabius plus SW 3.n...
-
Page 39: Steam Sterilization
– Check the locking device of the anesthetic vaporizer. – Check the function of the power failure alarm and the battery function. – Check the operation of the battery failure alarm. – Check S-ORC functionality. Supplement Fabius plus SW 3.n... - Page 40 Every 36 months Replace Experts Lead-gel battery Every 3 years Replace Experts Cylinder pressure reducer for After 6 years Basic overhauling Experts high-pressure cylinders Pressure reducer for PIN After 6 years Replace Experts index 1) optional Supplement Fabius plus SW 3.n...
-
Page 41: Technical Data
The conditions for use when using additional devices can limit the environment of use of a system as a whole. Vaporizers and anesthetic agents can limit the use of an anesthesia workstation with regard to its temperature range and maximum fresh-gas flow. Therefore when using additional devices, follow the associated instructions for use. Supplement Fabius plus SW 3.n... -
Page 42: Device Data
The following also apply for devices manufactured from July 2014 on: Ventilator The accuracy of the tidal volume has been changed: Accuracy Tidal volume (VT) ±5 % of the set value or 20 mL, whichever value is higher Supplement Fabius plus SW 3.n... - Page 43 ISO 21647 and ISO 80601-2-55 1) Max. ±4 % of the measured value or ±2 cmH O (±2 hPa), depending on which value is higher. Supplement Fabius plus SW 3.n...
- Page 44 No demonstrable quantitative effect on the gas measurement due to ambient conditions. Maximum period of use of O >12 months at 25 °C (77 °F), 50 % relative humidity, 50 % O in fresh sensor cell gas (or >5000 hours at 100 Vol% O Supplement Fabius plus SW 3.n...
-
Page 45: Breathing System
The alarm limit has changed: Alarm limit Warning signal (continuous tone for 10 seconds, adjustable from approx. 61 dB(A) to 74 dB(A)), as soon as the pressure drops below 20 ±6 psi (1.35 ±0.4 kPa x 100). Supplement Fabius plus SW 3.n... -
Page 46: Emc Declaration
"Environments of use" in the chapter "Application". Emissions Compliance Radiated emis- Class A, group 1 (30 MHz to sions 1 GHz) Conducted emis- Class A, group 1 (150 kHz to sions 30 MHz) Supplement Fabius plus SW 3.n... -
Page 47: Recommended Separation Distances From Wireless Communication Devices
Calibration of the sensors Calibrate the flow sensor. Remove the O sensor from the cover of the inspiratory valve. Expose the O sensor to ambient air for 2 minutes. Start the calibration. Supplement Fabius plus SW 3.n... - Page 48 Manufacturer Drägerwerk AG & Co. KGaA Moislinger Allee 53 – 55 D-23542 Lübeck Germany +49 451 8 82-0 +49 451 8 82-2080 http://www.draeger.com 9055305 – en Á9055305<È> © Drägerwerk AG & Co. KGaA Edition: 3 – 2018-07 (Edition: 1 – 2015-05) Dräger reserves the right to make modifications to the medical device without prior notice.















Need help?
Do you have a question about the Fabius plus and is the answer not in the manual?
Questions and answers