Atmos S 201 Thorax Operating Instructions Manual
Hide thumbs
Also See for S 201 Thorax:
- Operating instructions manual (68 pages) ,
- Quick start manual (4 pages) ,
- Quick manual (2 pages)
Summary of Contents for Atmos S 201 Thorax
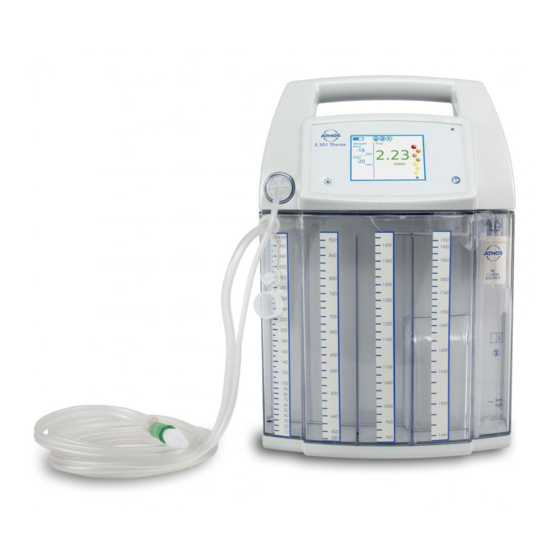
- Page 1 Operating Instructions ATMOS S 201 Thorax English These operating instructions are valid from software version 3.0.53 GA1GB.710201.0 0124 2021-10 Index: 42 E349855...
-
Page 2: Table Of Contents
Intended use ATMOS S 201 Thorax ........ - Page 3 Cleaning and care ..........50 8.1 General information on cleaning and disinfection .
-
Page 4: Introduction
These operating instructions must always be kept available near the device. Care and periodic tests in conjunction with professional execution provide for operational safety and readiness for use of your ATMOS S 201 Thorax and are therefore a must besides regular cleaning. Maintenance, repairs, and periodic tests may only be carried out by persons who have the appropriate technical knowledge and who are familiar with the product. The person in question must possess the necessary test devices and original spare parts required to carry out these measures. • This device bears the CE marking CE 0124 in accordance with § the European Medical Device Regulation (MDR) 2017/745. • The product ATMOS S 201 Thorax complies with all applicable requirements of the directive 2011/65/EC restricting the use of certain hazardous substances in electrical and electronic equipment (“RoHS”). • The Declarations of Conformity and our General Terms and Conditions can be viewed on our website at www.atmosmed. com. • The quality management system applied at ATMOS has been certified according to international standards EN ISO 13485. • Prior to starting up please peruse chapter “2.0 For your safety” on page 14 in order to be prepared for any possible dangerous situations. -
Page 5: Intended Use
1.2 Intended use 1.2.1 Intended use ATMOS S 201 Thorax Name: ATMOS S 201 Thorax Main function: The ATMOS S 201 Thorax is a device for mobile, digital cardiothoracic drainage. The system generates a controlled vacuum close to the patient and has an electronic monitoring system which shows the actual vacuum measured at the patient's side and the air leak. The objective therapy data is displayed in real time and illustrated in colour in a graph. Errors are automatically indicated by visual and acoustic warning messages. Intended use / intended Restoring the (natural) vacuum in the pleural cavity by purpose: draining off air and secretion. Intended users / user profile: •... -
Page 6: Intended Use Secretion Canister 2 L
Single-use product / • Options for reprocessing the device according to the reprocessing: operating instructions. • Secretion canister and hose system are disposable. For detailed information on the secretion canister and hose system, please refer to the separate intended uses. 1.2.2 Intended use secretion canister 2 l Name: Secretion canister 2 l Main function: The secretion canister transfers the controlled vacuum generated by the ATMOS S 201 Thorax. Fluids and air are drained through the secretion hose and collected in the secretion canister. The amount of fluid in the secretion canister can be read and documented on the balancing scales. An integrated bacterial and viral filter protects the device from possible contamination and from oversuction. In the event of excess pressure the pop-off valve opens immediately. An optional water lock is used to visualize the air leak on the patient's side by the appearance of air bubbles. Cover caps are used for proper sealing and disposal. Intended use / intended Collection of fluids and air from the thorax. -
Page 7: Intended Use Hose System
• Pleural empyema • Chylothorax • Other similar clinical pictures Medical contraindications: • Not suitable for cardiothoracic drainage therapy in which no vacuum should be applied to the patient. Other contraindications: • Do not use with cardiothoracic drainage systems other than the ATMOS S 201 Thorax. • No separate use of the secretion canister and the hose system (i.e., without the device) for short- or long-term gravity drainage (more than 60 minutes). • No application under emergency conditions. • Should not be used in the homecare sector which is not supervised by healthcare professionals. • No suction of flammable, corrosive or explosive fluids/ gases. - Page 8 Main function: The double lumen hose system transfers the controlled vacuum generated by the device. The secretion hose drains fluids and air into the secretion canister. The measuring and rinsing hose measures and regulates the vacuum applied on the patient's side. A bacterial and viral filter on the measuring and rinsing hose protects against contamination with bacteria and viruses. At defined time intervals, a valve opens in order to direct air through the measuring and rinsing hose into the secretion hose and to rinse liquids, coagulum and other blockages into the secretion canister. Chest tubes on the patient's side are connected to the hose system via the port. Intended use / intended Transport of fluids and air from the thorax. purpose: Measurement and regulation of the vacuum on the patient's side. Intended users / user profile: •...
-
Page 9: Function
Other contraindications: • No application with other cardiothoracic drainage systems other than the ATMOS C 051 Thorax, ATMOS S 201 Thorax and ATMOS E 201 Thorax • No separate application of the secretion canister and the hose system (i.e. without device) as gravity drainage. • No application under emergency conditions • Should not be used in the homecare sector which is not supervised by healthcare professionals • No suction of flammable, corrosive or explosive fluids/ gases Warnings: The following complications may occur during a cardiothoracic drainage: • Pain due to irritation of the intercostal nerves • Injuries to the lung parenchyma / air leak • Re-expansion edema • Effusion retention • Tension pneumothorax • Skin / soft tissue emphysema... -
Page 10: Transport And Storage
1.4 Transport and storage • The device may only be transported in a upholstered and protective shipping box. • Please note down and immediately report any damages which occurred during shipping. Please make use of the attached QD 434 Delivery complaint / Return shipment form if you have a complaint or send back the device. The form QD 434 can also be downloaded from our website www.atmosmed.com. • After transport of the device in temperatures below 0 °C or prior to first start up it should be kept at room temperature for at least six hours. If the device is not acclimatized it may not be used as damage to the diaphragms of the pump could occur. • Ambient conditions: Transport / storage: −10...+50 °C; 30...95 % air humidity without condensation at air pressure 700...1060 hPa Operation and battery charging: +10...+35 °C; 30...95 % air humidity without condensation at air pressure 700...1060 hPa Operation altitude: max. 3000 m 1.5 Explanation of pictures and symbols In the operating instructions DANGER Warning of a danger that will result in immediate fatal or serious injury. Observe the necessary measures. WARNING Warning of a danger that can cause fatal or serious injury. Observe the necessary measures. CAUTION Warning of a danger that can cause minor injury. Observe the necessary measures. - Page 11 · General information, numeration Sub-numeration Engage, check correct fit. click Follow the arrows Move, plug in this direction. Turn, push in this direction Please press where dot indicates Replace Check Symbols used for the ATMOS S 201 Thorax and its accessories Medical device This device complies with the relevant requirements of EU regulations. This device complies with the relevant requirements of EU regulations. 0124 UL Mark MEDICAL EQUIPMENT with respect to electrical shock, fire, and mechanical hazards only in accordance with E349855 UL60601-1/ ANSI/AAMI ES60601-1 (2005)/ CAN / CSA – C22.2 No. 60601-1 (2008) This device complies with the relevant requirements of the Eurasian Economic Union. GOST Certificate (Russia) Unique Device Identifier of a medical device Article number...
- Page 12 Serial number Batch code Use-by date Manufacturer Date of manufacture Country of manufacture: Germany Warning, pay special attention Consult operating instructions Follow operating instructions (blue) Do not reuse Sterile device STERILE Sterilized using ethylene oxide STERILE EO Non-sterile STERILE Double sterile barrier system Single sterile barrier system Protection class II device Applied part type CF Specification of the degree of protection against the ingress of solids and IP XO moisture Fuse...
- Page 13 Do not use if the packaging is damaged and follow the operating instructions. Fragile, handle with care Keep dry Keep away from sunlight Temperature limit Atmospheric pressure limitation Humidity limitation UDI application identifier (01) UDI-DI: Identification of the manufacturer and the device (10) Batch code (11) Date of manufacture (17) Expiry date (21) Serial number...
-
Page 14: For Your Safety
• When disconnecting the device from the mains power supply, pull the power plug first and then the device plug. • Disconnect the device from the mains power supply before cleaning or disinfecting it. • Never touch the plug or power cable with wet hands. • Never immerse the device in water or other liquids. • Do not take a shower / bath with the device! • The device is not sterilisable. • Use the power cable only in dry surroundings. The surroundings must be non-conductive. • Do not allow liquids (such as disinfectants or secretion) to enter the device or power cable. • Ensure that no liquid enters the device. If liquid gets into the device, operation of the device must cease immediately. In this case, clean and disinfect the device and send it to ATMOS for repair or an authorised service partner. • If disinfectant has penetrated the device, then it must be dried thoroughly and subsequently an efficiency control must be conducted. A check should be carried out to see whether the target vacuum is reached when the system is closed and whether a flow > 10 l/min is established after a while when the system is open. If not, it must not be operated until it has been checked by an authorised service partner or ATMOS Service centre. • Only use original accessories and original spare parts from ATMOS. This applies to the power cable in particular. • Follow the instructions regarding periodic tests in chapter ‘9.0 Maintenance and service’ on page 53. • Assembly, new settings, alterations, extensions, and repairs may only be carried out by... - Page 15 • Always wear disposable gloves if you could come into contact with secretion. • Clean and disinfect the device after every use. • Clean and disinfect the device according to the operating instructions. • The device must not be used following oversuction. WARNING Ensure that the device is always functional and ready for use Your patient can be severely injured. • Ensure that the device is always ready for use. • Place the device where it is easily accessible. • Perform a function check after each use. • ATMOS recommends always having another suction device ready at hand. This allows you to treat the patient and perform suctioning even in the event of device failure. • Please observe the notes on the electromagnetic compatibility (EMC) of the device. • If the device has fallen: Check the device for visible damage. A leakage test is recommended. If the leakage test fails or the housing is damaged, the device is defective and must not be operated. In this case, clean and disinfect the device and send it to ATMOS for repair. • The device and the secretion canister must always be used in a vertical position. If the device should tilt it must be placed upright again in order to guarantee faultless operation. If you are unsure whether the secretion canister works properly we advise you to replace the secretion canister to ensure the patients’ safety.
- Page 16 Luer lock cap for leakage. If there is still only a minimal flow value illustrated then there is an internal leakage in the system which cannot be rectified by the user. This will be compensated by the system but illustrated as a minimal flow value. • If the secretion canister tips over during temporary gravity drainage, the functionality of the water lock for gravity drainage is no longer guaranteed. The vacuum at the patient’s end ceases, thus posing a risk of a renewed pneumothorax. • In the case of larger air leaks and in patients with fistulas, the vacuum and thus the water column in the riser quickly drop to zero. This means that there is no vacuum at the patient’s end, thus posing a risk of a renewed pneumothorax. • If a high (negative) vacuum occurs (e.g. due to deep inhalation), the water in the water lock can rise through the riser. The vacuum at the patient’s end ceases, thus posing a risk of a renewed pneumothorax. • The device may not be operated in MRI scanners (magnetic resonance imaging). • The ATMOS S 201 Thorax is a medical device which is subject to special safety regulations. It must to be set up and put into operation in accordance with the EMC regulations. Portable and mobile RF communication devices (mobile phones) may affect the performance of the device. WARNING Avoid improper use. Your patient can be severely injured. • A misplaced drainage system and a misplaced chest tube could hinder the drainage of air and liquids. A complete blocking of the system during the drainage of liquids and air could cause a rise in pressure and thus lead to a tension pneumothorax. • Employ the device only according to its intended use. • The product may only be used by medically trained persons who have been instructed in the handling of the medical suction system. • Please select the vacuum according to the patient and the application. • Observe the valid guidelines.
-
Page 17: Avoiding Damage To The Device
• Faulty respectively damaged components must be replaced immediately. • A vacuum over -50 mbar could cause pain and injury to the patient. A vacuum over -50 mbar may only be adjusted under medical indication. WARNING Explosion and fire hazard. Burns and injuries are possible. • Only use original accessories and original spare parts from ATMOS. This applies to the power cable in particular. • Never operate the product in potentially explosive areas or in areas that are oxygenated. • Explosion-hazardous areas may be caused by the use of flammable anaesthetics, skin cleansing products and skin disinfectants. The ambient conditions specified in the technical data (chapter ‘11.0 Technical data’ on page 57) must be strictly observed. CAUTION Contact may cause allergic reactions! Your patient can be injured. • The materials used have been tested for their tolerability. In very rare cases, contact with accessible materials on the device and its accessories may cause allergic reactions. This applies in particular to contact injuries in conjunction with prolonged contact. If this occurs, seek medical attention immediately. CAUTION Tripping hazard due to cables. Injuries and fractures are possible. • Lay the power cable properly. -
Page 18: General Information
• The removal of the secretion canister from the device during the therapy may only be performed by trained professionals who act in conformity with guidelines. • A ready to use spare device incl. consumables must always be available. • The device supports the therapy of the patient it is not a substitute for the doctors‘ diagnosis. • The patient should be supervised constantly in accordance with the internal rules of the hospital. Electromagnetic compliance, - damage to the device! The device may become damaged. • The ATMOS S 201 Thorax fully complies with the electromagnetic immunity requirements of standard IEC 60601-1-2 / EN 60601-1-2 „Electromagnetic compatibility - Medical Electrical Devices“. Damage to the device due to improperly installed protective contact socket! The device may become damaged. • The ATMOS S 201 Thorax is designed in accordance with IEC 60601-1/EN 60601-1 and with protection class ll. • The device may only be connected to a properly installed protective contact socket. • Prior to first starting up, check whether the mains voltage specified on the type plate matches the local mains voltage. - Page 19 • Do not cover the device during suction. • Keep the device and the power cable away from other heat sources. • Do not place the device directly next to other devices as this may cause excessive heating of the device. • The device and the secretion canister should not be dried in a microwave oven. • The power cable and the device must be kept away from hot surfaces. • The device may only be operated at room temperature and should not be subjected to direct solar irradiation as this could lead to errors. • Do not close the ventilation slots on the bottom of the device. Otherwise the device could overheat. Exclusion of liability and warranty • no original ATMOS parts are being used, • the advice for use in these operating instructions is not being observed, • improper use, • assembly, new settings, alterations, extensions and repairs have been carried out by personnel not authorized by ATMOS. Advice on disposal • Dispose of wrappings accordingly. • Attention must be paid to all hospital protocols regarding disposal and infection control.
-
Page 20: Setting Up And Starting Up
3.0 Setting up and starting up 3.1 Scope of delivery The ATMOS S 201 Thorax was subjected to an extensive functional test and was carefully packed prior to dispatch. On receipt of the goods please check the packaging for any possible damage and compare the contents for completeness. (see bill of delivery). 312.1000.0 ATMOS S 201 Thorax 1x Basic device 1x Power cable , L = 3 m 507.0859.1 1x Operating instructions 1x Quick reference guide 3.2 Device overview Front side On / Off sensor Touchscreen (touch-sensitive display) Handle Light sensor... - Page 21 Rear side Type plate Charging socket Fixture for device attachment...
-
Page 22: Start Up
• Fuse: 1x T 1.25 A / H, 250 V 3.3.1 Battery charging Each bar of the symbol represents 20% battery charge. Attention! Prior to first start up of the ATMOS S 201 Thorax, the battery must be fully charged. The battery is recharged by the integrated recharging electronics as soon as you connected the device to the mains supply with the power cable. Please note the information on how to handle rechargeable batteries in chapter “9.4 Handling of batteries” on page 53. Correct handling of the rechargeable batteries prolongs the maximum service life. Batteries are wearing parts and therefore excluded from the general warranty. The device should be recharged in a cool place without direct solar irradiation. At ambient temperatures above 25 °C the charging time could be prolonged drastically. Defects which occur due to improper handling of the device are not covered by the guarantee. Attention: If the battery temperature is higher than 50° C it can no longer be charged. 1. Plug the recharger of the power cable into the charging socket of the ATMOS S 201 Thorax. 2. Plug the power plug into the socket. symbol on the display. � The ATMOS S 201 Thorax displays the The bar on the right side flashes. As long as the power plug is connected, the symbol is green. As soon as the battery is fully charged the last bar of the symbol stops blinking 3. Unplug the power plug from the wall socket. 4. Remove the recharger plug from the charging socket of the ATMOS S 201 Thorax. As soon as the battery charge level is less than 20%, the cardiothoracic drainage system displays a warning window and triggers an audible warning message (chapter “5.0 Warning messages” on page 43) Charge the battery in order to continue the therapy without interruption. If the battery is too low for further operation, the ATMOS S 201 Thorax switches off automatically. The battery of the ATMOS S 201 Thorax can also be charged when the device is switched off. The state of charge can be seen on the display. -
Page 23: Secretion Canister
3.3.2 Secretion canister • Always use the original ATMOS disposable secretion canister. • Vacuum connection system: The vacuum connection between device and secretion canister is established immediately when the secretion canister is engaged! Important safety • Due to hygienic reasons it is recommended to change the information on the hose system always together with the canister. secretion canister system 3.3.2.1 Secretion canister system overview - Versions with a water lock Only applicable for 312.1150.5 and 312.1120.0, not for 312.1140.0 (see chapter ‘7.0 Accessories, consumables and spare parts’ on page 48). - Page 24 3.3.2.3 Filling the water lock Only applicable for 312.1150.5 and 312.1120.0, not for 312.1140.0 (see chapter ‘7.0 Acces- sories, consumables and spare parts’ on page 48). The water lock is on the right side of the secretion canister. A bacterial and viral filter filter and a riser are included. The water lock is filled with water through the riser. For filling the water lock, a sterile cannula 20 G, a sterile syringe, and 60 ml sterile water are required. With the cannula you may puncture the silicone seal above the riser and then fill the water lock. Prior to use, the water lock must be filled to the prescribed filling level (not above the 2 cm level) and should be checked regularly to ensure proper operation. Obligatory: Temporary gravity drainage Optional: Normal operation, gravity drainage mode Prior to inserting the secretion canister into the device, make sure that the filler of the water lock is sealed with the plug. 5. Use only pre-packaged sterile fluid for filling the water lock. 6. Reconnect the filled canister to the device. 7. The canister may only be removed when the pump is switched off. 3.3.2.4 Pop-off valve The pop-off valve is a protective device against the occurrence of overpressure which could lead to a tension pneumothorax. The valve opens at an overpressure of ≥ 10 mbar within the rinsing canister. 3.3.2.5 Inserting the secretion canister Attention! Prior to use, check the packaging of the sterile products, the secretion canister system, and the hose system for intactness. Do not use defective secretion canisters or hose systems.
- Page 25 3.3.2.6 Changing the secretion canister Prior to exchanging the secretion canister the chest tube must be clamped so that a continuous vacuum is always available at the patient. Removing the secretion canister 1. Always wear disposable gloves, pay attention to the regulations for the handling of sterile products. 2. Provide a sterile secretion canister. 3. Check whether the target vacuum is reached. 4. Clamp the chest tube close to the fir tree connector so that a vacuum continues to be applied to the patient. 5. Stop the therapy. 6. Remove the secretion canister by pressing the blue release button and taking the secretion canister out of the guides on the left side. 7. Place the secretion canister securely on a horizontal surface. 8. Release the 2 luer lock connections by a counter-clockwise rotation to separate the secretion canister and the device from the hose system. Pay attention as secretion could be found in the connection space.
-
Page 26: Connecting The Hose System
3.3.3 Connecting the hose system Luer-Lock connection 4 mm with integrated hydrophobic bacterial and viral filter Measuring and rinsing hose Luer-Lock connection 6 mm Secretion hose Attention! Prior to use, check the packaging of the sterile products, the secretion canister system, and the hose system for intactness. Do not use damaged secretion canisters or hose systems. 1. Remove the sterile hose system from the sterile wrapping. 2. Connect the luer lock with the bacterial and viral filter to the upper canister connection on the secretion canister by rotating it clockwise. 3. Connect the luer lock connection with the larger diameter to the lower connection of the canister by rotating it clockwise. 4. A leakage test is recommended (chapter “A change of the bar chart with flow scaling from 450 ml/min to 150 ml/min takes place automatically as soon as the hourly average value is less than 150 ml/min. The recording is restarted in the process.” on page 30). 5. Use the sterile hose connector, supplied with the sterile fir tree connector, to connect the hose system to any drainage catheter of your choice. Alternatively you can also use conventional sterile y- or fir tree connectors. -
Page 27: Operation
4.0 Operation 4.1 Explanation of the display System information Display of the current fistula Attention: This is the current value. In the case of a discontinuous fistula, the value can decline temporarily to zero although the fistula still exists. Therapy process Changeover to the graphic diagram Actual vacuum Display of the actual User settings vacuum value. Target vacuum Setting dial Display of the Increase of the preadjusted target vacuum target vacuum to which the Setting dial pump adjusts Decrease of the... -
Page 28: Buttons And Display Symbols
4.2 Buttons and display symbols 4.2.1 Buttons Figure Function Decrease target vacuum Increase target vacuum Gravity drainage mode Graphic diagram of the therapy Open the user settings Save entry Confirm information Back / Exit menu Warning / suppress the warning Changeover to vacuum scaling Changeover to time scaling Changeover to flow scaling Start the therapy Stop the therapy Hold / restart graphic Increase maximum of axis Decrease maximum of axis Scroll up the list Scroll down the list Activate key lock... -
Page 29: Display Symbols
4.3 Explanation of the display in key lock modes 4.3.1 Key lock mode with bubbles In the key lock mode the flow is additionally displayed in traffic light coloured bubbles for at least one hour. Key lock activated Flow displayed as bubbles each additional colored bubble represents an additional flow. None: 0 - < 50 ml/min Green: 50 - < 100 ml/min Yellow: 100 - < 630 ml/min Orange: 630 ml - 5.51 l/min Red: >5.51 l/min to maximum. Up to 1.00 l/min the flow is displayed in ml/min. Day/Night Mode The ATMOS S 201 Thorax has a day/ night mode, i.e. the device adjusts automatically to the light conditions in a room. Under low ambient light conditions, the display has dark background illumination. 4.3.2 Key lock mode with bar chart If the key lock is active and the average flow value is less than 450 ml/min for at least one hour, the therapy process is displayed in a bar chart over 24 hours. The flow is visualised as an average value in bars on an hourly basis. The vacuum is displayed as a gradient line. -
Page 30: Switching On
The bar chart appears with a max. flow scaling of 450 ml/min if the average flow value is less than 450 ml/min for at least one hour. The bar chart appears with a max. flow scaling of 150 ml/min if the average flow value is less than 150 ml/min for at least one hour. A change of the bar chart with flow scaling from 450 ml/min to 150 ml/min takes place automatically as soon as the hourly average value is less than 150 ml/min. The recording is restarted in the process. If the hourly flow value exceeds the maximum of the flow scale, when the bar chart display is already active, then this bar is displayed in red. If the vacuum exceeds -30 mbar, the vacuum lines are no longer displayed. 4.4 Switching on 1. To switch on the ATMOS S 201 Thorax, touch the sensor above the symbol for 2 seconds. 2. The ATMOS logo appears with the software version number in the bottom right corner. 3. Depending on the user settings, the leakage test starts automatically after a short time (see next chapter ). 4. Subsequently, ‘Therapy progress’ will appear in the display. By pressing the buttons you can start a new therapy recording or continue recording. ) During initial start-up, only one new therapy can be started. 5. The main display appears. - Page 31 If the leakage test is activated, it will start automatically once the device is switched on. The hose attachment towards the drainage catheter should already be sealed with a sterile plug when starting up the device. Alternatively the chest tube can be clamped near to the patient. Do not clamp the ATMOS hose system. If the leakage test is error-free, the message „Leakage test OK“ appears. Now you can remove the plug from the hose. By pressing the button you will reach the main menu. If the leakage test is faulty, the message „Leakage test failed“ appears. Check the hose connections and whether the secretion canister is correctly clicked into place. By touching the corresponding buttons you now have the option to. a) repeat the test b) abort the test and continue by pressing the respective buttons on the screen. ATTENTION: If the leakage test is operated accordingly, the leakage must not be ignored. If the device has been dropped, it must no longer be operated. Send the device in for repair. Treatment with defective device can...
-
Page 32: Function
4.6 Function 4.6.1 Target vacuum • Please note, an adjusted target vacuum over -50 mbar may cause pain and injuries to the patient. • On the main screen the target vacuum can be set directly by pressing the buttons. • ATTENTION: The change in the target vacuum takes effect immediately. There is no confirmation necessary. • The target vacuum can be freely selected between -5 and -100 mbar in steps of 1 mbar. • If the buttons are pressed permanently, the increase / decrease is accelerated • The target vacuum of -20 mbar is preset when starting the device. -
Page 33: Suction
• The settings of the target vacuum from the gravity drainage mode can be changed again using the symbol 4.6.3 Suction • When the device is switched on the pump is not activated. The pump or therapy will be started by pressing the button. This is visually illustrated by a symbol change from in the lower left of the display. • By pressing the button the pump will be stopped. • The ATMOS S 201 Thorax has a vacuum regulator. On the one hand this means, that the integrated pump only starts, if the actual vacuum does not correspond to the target vacuum. On the other hand the pump performance depends on the difference between the actual vacuum and the target vacuum. • The vacuum is measured at the patient-side of the hose system. -
Page 34: Key Lock
4.7 Key lock The ATMOS S 201 Thorax has an automatic key lock. 1. Automatic activation of the key lock If the settings on the display are not changed for a defined time, the key lock will be activated automatically (default factory setting 1 minute, individually adjustable in the user settings). This will prevent unintentional operation. 2. Manually activate the key lock The key lock can be manually activated after all the therapy values are set and the therapy is started. Press the symbol to activate the key lock. The display symbol appears above the Flow readout and shows an activated key lock. 3. Deactivate the key lock A quick touch to the display and then the first contact point appears By touching the contact point the second contact point appears By a repeated touching of this contact point the key lock symbol above is deactivated (see first display image). Now you can operate the system again. If you do not touch both the symbols within 6 seconds the key lock remains... -
Page 35: Therapy Process
4.8 Therapy process The ATMOS S 201 Thorax offers 2 graphs to simplify the analysis of the flow and actual vacuum process. Selection menu By selecting the buttons you enter the menu for graphical diagram modus. By pressing the buttons you can select the modus of your choice e.g. long time display / short time. 4.8.1 Short time display The graphical diagram starts by selecting the menu. In this modus the real measurements (flow, vacuum) from the last 30 seconds can be shown. Therefore you can visualise cough tests and other proceedings. By pressing the button the diagram can be frozen to enable a graphical interpretation. When you press the button again the short time display time diagram is restarted. By pressing the button you will return to the main menu. ) Set the period duration of the hose rinsing to > 5 minutes if you want to use the short time display for real-time display of the flow, such as for cough tests, to detect blocked catheters, etc. 4.8.2 Long time display In the long time display the complete therapy process can be visualised. - Page 36 When changing the flow scaling in the long time display to the smallest scaling, the message appears that the scaling unit has changed from l / min to ml / min. If you have initially set the zoom values and go back to the long time display, the previously set zoom values are saved, even if the device has been switched off in the meantime. ) If the recorded therapy data is greater than the set scaling for the long time display, then this is not displayed in the usual lines. The target vacuum is recorded in light blue. The actual vacuum is recorded in dark blue. The flow value is recorded in green.
-
Page 37: Transfer Of Therapy Data
4.8.3 Transfer of therapy data You may transfer the therapy data to a USB flashdrive. The therapy data is saved as a PDF- and Excel-file. If you continue the therapy after the data transfer, the data will still be recorded. The transmitted data will not be deleted. If you are starting a new therapy, the previous data will be overwritten. ATMOS recommends: Perform the therapy data transfer at the end of the patient's therapy. Suitable USB flash drives for therapy data transfer • Manufacturer: SanDisk, Kingston, ATMOS flash drive • System: USB 2.0, 3.0, 3.1 • Capacity: ≤ 32 GB • Formatting: FAT 32 • No stored encryption ATMOS recommends: Use a USB flashdrive without content. Other USB flash drives may not be recognized, thus the therapy data read-out does not start. Start the therapy data transfer •... -
Page 38: Reading Out The Therapy Data
Complete data transfer • As soon as the therapy data is transferred then the USB flashdrive may be removed. Now you return to the main screen. If the therapy data should be transferred during a patients therapy, follow the steps below: • Clamp the chest tube • Stop the current therapy • Remove the secretion canister Perform the therapy data transfer as described. • Connect the secretion canister • Continue the therapy • Open the clamp at the chest tube 4.8.4 Reading out the therapy data • Connect the USB flashdrive to the PC. •... -
Page 39: User Settings
4.9 User settings Press the symbol to access user settings. Press the buttons for moving up and down in the menu selection. To select a settings menu, press the text box. These buttons can be found in every settings menu: • By pressing the button you will return to the user menu. • The selected data are only saved if you press the memory key... - Page 40 In the user settings the following positions can be selected: The system language can be System language adjusted with When the device is started the standard vacuum is automatically pre-adjusted. Standard vacuum You can adjust the standard vacuum with You can change the cycle Cycle duration for hose duration for hose rinsing with rinsing The vacuum unit can be Vacuum unit adjusted with Leakage Test The leakage test can be Leakage test activated or deactivated with...
- Page 41 WARNING The warning message ‘Device Critical tilt Warning in critical tilt’ can be activated or deactivated with Critical tilt The key lock activation time Key lock activation time can be adjusted with The key tone can be activated Key tone or deactivated by pressing By pressing one of the two buttons (hour or minute) you can access the individual settings. Time Now you can change the time with By pressing one of the three buttons (Day, Month, Year) you can access the individual settings. Date Now you can change the date with...
-
Page 42: Switching Off The Device
The user settings can be locked in the service menu if required. If the setting is Unlocking the user settings activated, the user settings via key code can only be unlocked and operated using a corresponding key code. 4.10 Switching off the device • To switch off the ATMOS S 201 Thorax stop the therapy and touch the sensor for 2 seconds. • The ATMOS logo appears on the screen and the device shuts down. -
Page 43: Warning Messages
"Secretion canister full or hose patient catheter blocked" is activated. - Secretion hose system Possible reasons for this error to the secretion canister message are: - Secretion canister Leakages connection • Contact the ATMOS service If the target vacuum is not • Check for blockages: achieved, the warning message - Secretion canister "Vacuum too low" and - Hose "Secretion canister full or hose - Filter in the secretion blocked" is activated. canister... - Page 44 If the warning message is "Inactive therapy" appears with suppressed, it appears again tone. with tone after one minute. The device must no longer be Contact the ATMOS service. operated. Possible causes: • Battery or • pump defective. Device temperature too high. • Place the device in a cooler location. • Device is in the sun or near a WARNING heater.
- Page 45 Display Cause Recommended actions Carry out an inspection Contact the ATMOS service. according to the manufacturer‘s specifications every 12 months. This will be displayed on the device. A dwindling battery capacity is Battery must be replaced by indicated to you on the device. the service. If the set target vacuum is over -50 mbar the notice "High target vacuum is set” appears. When changing the flow scaling in the long time display to the smallest scaling, the notice indicating that the scaling unit has changed from l / min to ml / min. appears.
-
Page 46: Function
6.0 Function 6.1 Hose rinsing • The ATMOS S 201 Thorax has an automatic hose rinsing function which works periodically. • The rinsing process transports secretion located in the secretion hose into the secretion canister. • The rinsing process is initiated by opening a valve located in the measuring and rinsing hose. • The manufacturer's default setting for the period between 2 rinsing cycles is 3 minutes. If the water lock function is being used, air bubbles are likely to appear during the hose rinsing period. These air bubbles occur at regular intervals according to the set period duration of the hose rinsing and have nothing to do with the patient's condition (e.g. fistula). The automatic hose rinsing is indicated by the symbol in the display. 6.2 Gravity drainage mode while using the drainage system During normal operation, the filling of the water lock in the secretion canister is optional. A physiological vacuum can be generated by setting the target vacuum to -5 mbar (press the symbol The automatic warning messages as well as all measuring functions and hose rinsing are retained. Thus, the physiological vacuum in the thorax is maintained while preserving the digital safety features. The drainage system must be positioned at the same height as the patient catheter. - Page 47 7. The vacuum applied at the patient’s end corresponds to the water level in the riser of the water lock. 8. The secretion hose and water lock must be inspected regularly by qualified medical staff to ensure correct operation.
-
Page 48: Accessories, Consumables And Spare Parts
7.0 Accessories, consumables and spare parts Accessories Universal bracket for the ATMOS S 201 Thorax 312.1160.0 Carrying strap for the ATMOS S 201 Thorax 312.0850.0 Hose clamp 061.0079.0 Consumables Surgical kit for ATMOS E / S 201 Thorax 312.1031.0 Included in thesurgical kit: Secretion canister 2 l, 10 pcs. (sterile) Hose system, 10 St. (sterile) Hose system, 10 pcs. 312.1170.0 Secretion canister 2 l, 5 pcs. 312.1150.5 Secretion canister 2 l without water lock, 5 pcs. 312.1140.0 Secretion canister 2 l — standard, 10 pcs. 312.1120.0 Y-port, 50 pcs. 312.1101.0 Paediatrics port, different sides, 1 pcs. 312.1102.0 Fir tree port (without luer lock), 1 pcs. 312.1103.0 Connection set for thorax chest tube, 50 St. 312.1104.0 Spare parts Power cable , L = 3 m 507.0859.1 Power cable , L = 5 m... -
Page 49: Attachment Of The Universal Bracket (Accessories)
7.1 Attachment of the universal bracket (Accessories) The universal bracket can be mounted to infusion tripods, wheelchairs, to crossrods and longitudinal bars of the bed or to the standard rail. Align the fastening bracket 1. Pull the stop bolt 2. Rotate the fastening bracket 90°. 3. Allow the stop bolt to snap into position. Attach the universal bracket: 4. Position the universal bracket to the required position. 5. Turn the knob until the universal bracket is fixated. Attach the device to the universal bracket: 6. Pull the locking system rotate it by 90° so that the stop bolt is drawn in 7. -
Page 50: Cleaning And Care
8.0 Cleaning and care 8.1 General information on cleaning and disinfection Prior to cleaning: Medical devices such as the ATMOS S 201 Thorax must operate safely and reliably at all times. Therefore we recommend prior to every use: if required Handling of the cardiothoracic drainage system determines to a large extent its reliability and safety. The hygiene measures are necessary measures for the protection of patients and users, and to maintain functional reliability of the cardiothoracic drainage system. Prior to cleaning the device please remove all disposable parts such as secretion canister and hoses. Please remove the power cable. The described actions relating to cleaning and disinfection or sterilization do not substitute the relevant instructions which must be adhered to prior to operation! Some disinfectants could cause discolouring to some of the plastic parts. Avoid the penetration or liquid entering the cardiothoracic drainage system, especially in the connections on the rear side of the device. Please observe the operating instructions for use prescribed by the manufacturers of disinfectants. Pay attention regarding their concentration suitability for use and the contact time. Do not use • Disinfectants which contain organic or inorganic acids or bases as they could cause corrosion damage. • Disinfectants containing chloramides or phenol derivatives, since these may cause stress cracks in the plastic material used. During all work disposable gloves must be worn. For disinfection, you may use all surface disinfectants listed in chapter “8.4 Recommended disinfectants” on page 51. -
Page 51: Cleaning The Device Surface
The device must never be autoclaved, rinsed under running water or immersed into any liquids. 8.3 Cleaning the device surface Prior to using the device on a new patient the complete device surface must always be cleaned with a damp (not wet) cloth and disinfected with a surface disinfection solution. In case the device is being used by the same patient the surface should still be cleaned at least once every week with a damp (not wet) cloth and afterwards be disinfected with a surface disinfectant. • Attention! The device should never be autoclaved, rinsed under running water or immersed into any liquids! 8.4 Recommended disinfectants Disinfectant Contents in 100 g Manufacturer ATMOS Green & Clean SK Di alkyl dimethyl ammonium chloride < 1 g Metasys, Rum Alkyldimethylethylbenzylammoniumchloride < 1 g (Austria) Alkyldimethylbenzylammoniumchloride < 1 g Dismozon® pur Magnesium peroxyphthalate 80 g Bode Chemie, (granule) Hexahydrate Hamburg End of product 12/2014 Dismozon® plus... -
Page 52: Hygiene Plan
8.5 Hygiene plan WHAT WHEN Notices Manual wipe cleaning Device Manual wipe disinfection Disposable product — not Canister 2 suitable for reprocessing, change after use Disposable product — not Hose system 2 suitable for reprocessing, change after use A new carrying strap should be Carrying strap used for each patient. Disposable product — not Ports 2 suitable for reprocessing, change after use Manual wipe cleaning Universal bracket Manual wipe disinfection R = Removal, C = Cleaning, D = Disinfection, S = Sterilization... -
Page 53: Maintenance And Service
• Operational and functional errors that cannot be resolved by means of the measures described in the chapter ‘“10.0 Troubleshooting” on page 55’. 9.3 Sending in the device If the cardiothoracic drainage system has to be sent in for repair after consultation with the manufacturer or an authorised service partner, we ask you to observe the following: 1. Remove all consumables and dispose of them properly. 2. Clean and disinfect the product and accessories in accordance with the operating instructions. 3. Place any used accessories with the product. 4. Fill in form QD 434 “Delivery complaint / return shipment” and the corresponding Decontamination certificate. This form is enclosed with each delivery and can be found at www.atmosmed.de. 5. The device must be well padded and packed in suitable packaging. 6. Place the form QD 434 “Delivery complaint / return shipment” and the respective decontamination certificate in an envelope. 7. Affix the envelope to the outside of the package. 8. Send the product to ATMOS or your dealer. 9.4 Handling of batteries Rechargeable batteries are wear parts with a limited lifetime. Under optimal conditions of use, lithium-ion batteries are usually worn after approx. 500 charge cycles and should then be replaced. Handling of the device and the batteries significantly affects lifetime of the batteries. Non-observance of the following recommendations may significantly decrease lifetime. -
Page 54: Changing The Fuse
) Always store device with batteries in a cool and dry place (room temperature 18 - 25° C). ) Always store device with batteries at a charge status of 20–40%. ) Avoid deep discharge: Devices with permanently installed batteries should be recharged every 4-5 months. ) Never cover the device, never expose the device to direct sunlight and never charge, operate or store the device in close vicinity of heaters. ) Always charge the batteries using the respective charging accessories. Overcharging will destroy the batteries. ) The lifetime of lithium-ion batteries mainly depends on the ambient temperature. On principle batteries are depleted after 2.5 years. ) New batteries should be fully charged prior to first use. ATMOS has no influence on the use of the device therefore batteries are excluded from the guarantee. There is a function guarantee of 6 months. Using other charging accessories may result in risk of explosions! 9.5 Changing the fuse click... -
Page 55: Troubleshooting
Check the bacterial and viral filter on the measuring hose and in the secretion canister. If the bacterial Bacterial and viral filter and viral filter on the measuring blocked on the measuring hose is blocked, replace the hose hose or secretion canister. system. If the bacterial and viral filter in the secretion canister is blocked change the secretion canister. Contact ATMOS Service or a certified service partner. Liquid sucked into pump. The device must be checked. ‘Vacuum too high’ Excessively high vacuum Check for correct hose connections. applied from the outside. Ventilation valve is defective. Contact ATMOS Service or a certified service partner. The device must be checked. ‘Battery low’ Battery almost empty. Connect device to the supply network. The battery is charged and the state of charge is indicated in the display. - Page 56 System shut down. Battery empty. Connect device to the supply network. The battery is charged and the state of charge is indicated in the display. High temperature of the Ventilation slots are covered. Please ensure sufficient air device ventilation. Contact ATMOS Service or a certified service partner. The device must be checked. Leakage test failed. Hose system is not Check the hose system and rinsing canister for correct fit. See ‘3.3.2.6 completely closed. Changing the secretion canister’ on Secretion canister is leaking. page 25 Contact ATMOS Service or a certified Internal error. service partner. The device must be checked. Flow readout is always in Component error 1) Check whether the flow is also 0 l/ 0 l/min. min when the system is open.
-
Page 57: Technical Data
Other safety equipment „Pop-off valve“ in the rinsing canister Vacuum in the device limited to approx. 150 mbar. Acoustic and optical error warnings. Pump performance Free flow 18 ± 2 l/ min. Vacuum adjustable from -5 mbar to -100 mbar, step size -1 mbar. Display Graphic display, colour, with background lighting, display of target vacuum and actual vacuum in mbar, cmH O, kPa and flow in l/ min. Data memory Internal memory for therapy data: 2.5 MB. Up to 12 days recording possible. Canister ATMOS disposable canister, transparent, with integrated water lock, pop-off valve, graduation. Max. volume of 2 l, connection to the device with“Direct-Dock- ing-System“. Material: PC. Suction hose ATMOS disposable suction hose for thorax, double lumen, with integrated bacterial and viral filter in the measuring channel, 180 cm length. Operating time Continuous operation in the specified temperature range. Simultaneous battery recharging and operation possible. Battery operation time at maximum continuous suction Battery operation time... -
Page 58: Bacterial And Viral Filter
Type of protection IP X0 Classification in Class IIa accordance with Annex IX of EC Directive 93/42/EEC CE marking CE 0124 GMDN code 36787 UMDNS code 10-218 Suction device, thoracic ID no. (REF) 312.1000.0 ATMOS S 201 Thorax 11.1 Bacterial and viral filter Bacterial filtration efficiency (BFE) 99.999778%* Viral filtration efficiency (VFE) 99.73 %* Overall filtration efficiency >99.95%* Filter class H13 (High-Efficiency Particulate Air/Arrestance)* *External test report (test laboratory) Status of the Technical Data: 16.06.2016... -
Page 59: Disposal
12.0 Disposal • Please observe national disposal regulations (e.g., waste incineration). • Device and accessories must be decontaminated prior to disposal, as secretion residuals could pose a danger to a third party. • Pay attention to a careful separation of the different materials. • The housing material is fully recyclable. • The ATMOS S 201 Thorax has a lithium-ion battery which must be disposed of in accordance with existing directives. Disposal within the EU The device described above is a high-quality medical product with a long service life. After its life cycle, it must be disposed of professionally. According to the EC directives (WEEE and RoHS) the device may not be disposed of with domestic waste. Please observe existing national laws and rules for disposal of old devices in the respective country. Disposal within the Federal Republic of Germany In the Federal Republic of Germany, the law for electrical devices (ElektroG) regulates the disposal of electrical devices. It must be assumed that such suction devices can be contaminated. Therefore, according to the regulations of the EAR foundation (Used Electrical Appliances Register), this type of device is excluded from ElektroG regulations. In order to guarantee proper disposal of your old device, please either pass it on to your specialised dealer or send it directly to ATMOS MedizinTechnik GmbH & Co. KG for professional disposal. Prior to disposal or before transport, secretion canister and all hoses must be removed. -
Page 60: Notes On Emc (Electromagnetic Compatibility)
13.2 Guidance and manufacturer’s declaration – key features The ATMOS S 201 Thorax has the following electrical components: Type Max. cable length Power cable , L = 3 m 507.0859.1 3 m Power cable , L = 5 m 008.0629.0 5 m 13.3 Guidance and manufacturer’s declaration – warnings WARNING The use of accessories, transducers, and cables other than those specified or provided by the manufacturer may cause increased electromagnetic emissions or reduced immunity to electromagnetic interference and result in faulty operation. WARNING Portable HF communications equipment (radios, antenna cables) should not be used within 30 cm* of any parts as specified by the manufacturer or of the ATMOS S 201 Thorax cables. Otherwise, degradation of the performance of this device could result. *The distance may be reduced at higher immunity test levels. WARNING Avoid using the ATMOS S 201 Thorax in close proximity to other devices or with other devices in a stack, as this may cause interference. If this cannot be avoided, the device must be monitored regularly for proper functioning and If possible please switch off any nearby devices that are not in use. - Page 61 For your notes...
- Page 64 ATMOS MedizinTechnik GmbH & Co. KG Ludwig-Kegel-Str. 16 79853 Lenzkirch / Germany Phone: +49 7653 689-0 info@atmosmed.de www.atmosmed.com...














Need help?
Do you have a question about the S 201 Thorax and is the answer not in the manual?
Questions and answers