Table of Contents
Advertisement
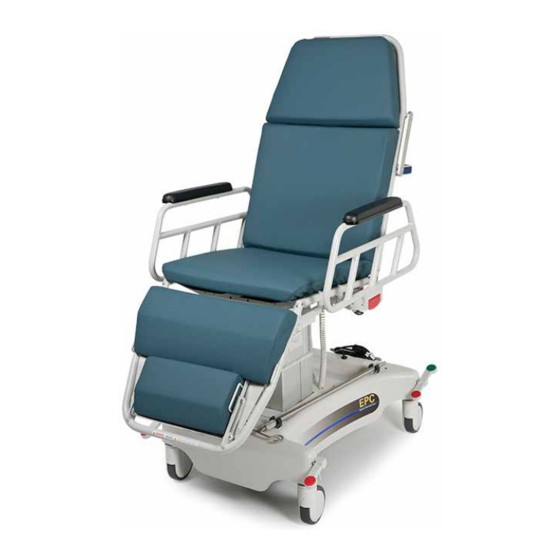
APC250 Hydraulic
EPC Electric
ALL PURPOSE CHAIRS
HYDRAULIC APC250 AND
ELECTRIC EPC, EPD, ESC, AND ESD MODELS
OPERATING MANUAL
READ THIS MANUAL BEFORE OPERATING YOUR APC250 / SURGI-CHAIR.
SAVE THIS MANUAL FOR FUTURE USE.
THE MOST CURRENT VERSION OF THIS MANUAL CAN BE FOUND ONLINE AT
WWW.HAUSTED.COM.
APC-US-INS-LAB-REVC18
Advertisement
Table of Contents
Troubleshooting

Summary of Contents for Graham Field HAUSTED EPC Series
- Page 1 APC250 Hydraulic EPC Electric ALL PURPOSE CHAIRS HYDRAULIC APC250 AND ELECTRIC EPC, EPD, ESC, AND ESD MODELS OPERATING MANUAL READ THIS MANUAL BEFORE OPERATING YOUR APC250 / SURGI-CHAIR. SAVE THIS MANUAL FOR FUTURE USE. THE MOST CURRENT VERSION OF THIS MANUAL CAN BE FOUND ONLINE AT WWW.HAUSTED.COM.
-
Page 2: Table Of Contents
CONTENTS INTRODUCTION — A WORD FROM GF HEALTH PRODUCTS, INC...................... 4 INDICATIONS FOR USE ................................4 SERVICE INFORMATION ................................. 4 ADVISORY ....................................4 LIST OF WARNINGS AND CAUTIONS ............................. 5 SIGNIFICANCE OF SAFETY STATEMENTS ............................ 5 DANGER / WARNING / CAUTION / NOTICE SUMMARY ......................5 WARNING: TO REDUCE THE RISK OF BURNS, FIRE, ELECTRIC SHOCK, OR PERSONAL INJURY .... - Page 3 3.5.7 HYDRAULIC MODEL: TOP ADJUSTMENT ........................ 15 3.5.8 HYDRAULIC MODEL: TRENDELENBURG ADJUSTMENT ..................15 3.6 HYDRAULIC MODEL: INDEPENDENT FOOT SECTION OPERATION ................16 3.6.1 INDEPENDENT OPERATION ............................16 3.6.2 RETURN TO CHAIR MODE ............................16 3.7 ADJUSTABLE FOOTREST ..............................16 3.7.1 REPOSITIONING THE FOOTREST ..........................
-
Page 4: Introduction - A Word From Gf Health Products, Inc
Manufactured by: Class 1 Equipment Type B Equipment GF Health Products, Inc. One Graham-Field Way Equipment not suitable for use in the presence of flammable anesthetic mixture Atlanta GA 30340-3140 with air or oxygen or nitrous oxide. -
Page 5: List Of Warnings And Cautions
Always consult your healthcare professional to determine safe methods most suitable for your individual abilities. Protect yourself, your attendant, and the Hausted APC / Surgi-Chair by having it serviced regularly. If you experience any malfunction, contact your Graham-Field authorized distributor immediately, as a hazardous condition could result, causing personal injury or damage to the Hausted APC / Surgi-Chair. -
Page 6: Warning - Cautions And Proper Operation
WARNING — CAUTIONS AND PROPER OPERATION WARNING: The APC250 model chair has a maximum weight capacity of 325 lb (147 kg), EVENLY DISTRIBUTED. The EPC, EPD, ESC, and ESD model chairs have a maximum weight capacity of 500 lb (227 kg), EVENLY DISTRIBUTED. WARNING: The chair is not intended to replace a stretcher or gurney. -
Page 7: Electromagnetic Compatibility (Emc) Information
WARNING: This product can expose you to chemicals including Di(2-ethylhexyl)phthalate (DEHP) which is known to the State of California to cause cancer or birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov/furniture. ELECTROMAGNETIC COMPATIBILITY (EMC) INFORMATION WARNING: Medical Electrical Equipment needs special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided in this manual. -
Page 8: Uncrating Instructions
UNCRATING INSTRUCTIONS IMPORTANT — REPORT ANY SHIPPING DAMAGE IMMEDIATELY: WARNING: Inform shipper of any damages — leave carton intact. Leave equipment in the receiving area until inspection is complete. NOTICE — POSSIBLE EQUIPMENT DAMAGE: s NOTICE: The crate contains fragile, expensive medical equipment. Uncrate and handle carefully. -
Page 9: Operating Instructions
OPERATING INSTRUCTIONS APC / SURGI-CHAIR SPECIFICATIONS Info: All dimensions are specified in inches. Unless otherwise noted, all dimensions are ±.375. Stacked dimensions are minimum (left) and maximum (right). GF Health Products, Inc. reserves the right to change specifications without notice. 20.0 DIMENSION 6 TRENDELENBERG... -
Page 10: Features, Warnings And Proper Operation Operating Instructions
FEATURES, WARNINGS AND PROPER OPERATION OPERATING INSTRUCTIONS WARNINGS — CAUTIONS AND PROPER OPERATION (See Diagram on following page) WARNING: The APC250 model chair has a warning label located on the back section stating: Maximum patient weight 147 kilograms (325 lbs.). The EPC, EPD, ESC, and ESD model chairs have a warning label located on the back section stating: Maximum patient weight 227 kilograms (500 lbs.). -
Page 11: Features (Shown In Illustration)
WARNING: The chair has a warning label located on both side rails indicating a pinch point between seat section and side rail. Features (Shown in Illustration) HEAD SECTION TUBE WARNING LABEL See Warnings C & H PATIENT COMFORT CUSHIONS BACK OF CHAIR WARNING LABEL See Warning A PATIENT ENTRY See Warnings G &... -
Page 12: Braking And Steering Operation
BRAKING AND STEERING OPERATION 3.3.1 Applying the Brakes Activate the four wheel central braking system by depressing the red pedal at any of the four corners of the unit (Figure 3-1). Engage the brakes fully by depressing the pedal to approximately 45°. All four caster wheels FIGURE 3-1 should then be locked from swiveling and rotating. -
Page 13: Electric Control Locations
ELECTRIC CONTROL LOCATIONS 3.4.1 Pendant Control Location The pendant is located in the pendant holder on the back of the chair (Figure 3-4). This chair is equipped with a battery back up for transport but the unit should be plugged into a wall receptacle when not in transport. -
Page 14: Height And Top Adjustment
HEIGHT AND TOP ADJUSTMENT 3.5.1 Electric Models: Height Adjustment Remove the pendant from pendant holder, (see Section 3.4.1, Pendant Control ). Raising the Height Press the first button on the third row of buttons on the pendant (Figure 3-7); hold until the FIGURE 3-7 desired height is achieved. -
Page 15: All Models: Quick Drop Activation
HEIGHT AND TOP ADJUSTMENT 3.5.4 All Models: Quick Drop Activation This chair is equipped with a manual override function for the back section of the chair. This option should only be used in an emergency situation. To activate the quick drop, support the back section and pull upward on the red activating handle located on the back of the FIGURE 3-13... -
Page 16: Hydraulic Model: Independent Foot Section Operation
HYDRAULIC MODEL: INDEPENDENT FOOT SECTION OPERATION 3.6.1 Independent Operation To adjust the foot section separately from the back section, support the foot section with one hand, pull out the release lever, then turn it clockwise (Figure 3-17). Adjust the foot section to the desired position and release the lever. -
Page 17: Hinging Rails
HINGING RAILS 3.8.1 Repositioning the Rail The chair rail has two positions, up and down. Both positions lock the rail into place. To lower the rail, apply a lateral force to the top of the rail (Figure 3-21) and pull on the red rail handle (Figure 3-22). -
Page 18: Esceye / Esdeye Headrest
ESCEYE / ESDEYE HEADREST 3.9.1 Pre-op / Post-op Head Extension To remove the head extension, rotate the knobs on the two ends of the extension counterclockwise (Figure 3-24). With knobs FIGURE 3-24 loose, pull out on the extension. To install the extension, slide the extension over the surgical mounting bars using the guides on the extension. -
Page 19: Common Optional Accessories
3.10 COMMON OPTIONAL ACCESSORIES 3.10.1 Mounting the Wrist Rest Insert the wrist rest into one of the appropriate three square sockets under the headrest (Figure 3-31). Rotate the T-knob on the back of the wrist rest (Figure 3-32) clockwise to lock it into place. FIGURE 3-31 s NOTICE: Ensure the wrist rest is secure before applying any pressure. -
Page 20: Using Restraint Straps
3.10.5 Using Restraint Straps Locate both ends of the restraint strap, on either side of the chair. One will have a square loop on the end, and the other will have hook and loop fasteners (Figure 3-37). Slide the end of the strap with the hook and loop fasteners through the loop on the other strap (Figure FIGURE 3-37... -
Page 21: Troubleshooting Guide
TROUBLESHOOTING GUIDE ELECTRIC POWERED CHAIRS TROUBLESHOOTING DANGER: SHOCK HAZARD — To reduce the risk of electric shock, do not remove the cover. Unit is to be serviced by qualified service personnel (minimum 1 year medical equipment service and repair experience) only. DANGER: SHOCK HAZARD —... -
Page 22: Hydraulic Chairs Troubleshooting
HYDRAULIC CHAIRS TROUBLESHOOTING The following list of problems and their solutions will assist you in determining what may be causing your chair not to function as designed. Then Unit doesn’t lower Column is adjusted too tight — adjust column. when pedal is depressed Unit is unstable at Column is adjusted too loose —... -
Page 23: Preventive Maintenance For The User
PREVENTIVE MAINTENANCE FOR THE USER Component Cleaning Procedure Schedule Cleaning Agent * Special Notes Pads / Wipe with damp cloth to Routine hospital grade Use only medium Mattresses remove any foreign material disinfectants, soap and strength cleaners water Do not steam clean or After pressure wash each... -
Page 24: Optional Accessories
OPTIONAL ACCESSORIES WARNING: Use only accessories approved by GF Health Products, Inc. with this device. The use of accessories, transducers, and cables other than those specified by the manufacturer may result in increased emissions or decreased immunity of Hausted equipment. Info: To order accessories, or for more detailed information on accessories, please contact GF Health Products, Inc. - Page 25 Universal Accessories Continued ESC / ESD EPC / EPD TRAY / SIDE TABLE H06892700 FOLDING SIDE TABLE ASSEMBLY X - PRE 3/2018 X - PRE 7/2012 X - PRE 7/2012 H080346 FOLDING SIDE TABLE ASSEMBLY X - POST 3/2018 X - POST 7/2012 X - POST 7/2012 HAPC02100 PATIENT TRAY W/ STORAGE RACK...
-
Page 26: Gf Health Products, Inc. Limited Warranty For Hausted Brand Product Line Within The U.s
GF HEALTH PRODUCTS, INC. LIMITED WARRANTY FOR HAUSTED BRAND PRODUCT LINE WITHIN THE U.S. SCOPE OF WARRANTY GF Health Products, Inc. (“GF”) warrants to the original purchaser only that it will replace or repair components, at GF’s sole discretion, that are defective in material or workmanship under normal use and service. All warranties are conditioned upon the proper use of the products strictly in accordance with good commercial practice and applicable GF instructions and manuals, including proper use and maintenance. -
Page 27: Disposal And Key To Symbols
DISPOSAL AND KEY TO SYMBOLS DISPOSAL Hausted equipment and accessories can be disposed of. We recommend disassembling and dividing the equipment and components into different waste groups such as: metal, cable, electronic, recoverable resource and plastic for recycling or combustion. Most plastic components are provided with a plastic types code and fiber content to aid sorting of plastic parts. -
Page 28: Appendix
APPENDIX GUIDANCE AND MANUFACTURER’S DECLARATION — ELECTROMAGNETIC EMISSIONS The Hausted APC Series Models and Surgi-Chairs are intended for use in the electromagnetic environment specified below. The customer or the user of the Hausted APC Series Models and Surgi-Chairs should assure that they are used in such an environment. -
Page 29: Enclosure Port Immunity To Rf Wireless Communications Equipment
ENCLOSURE PORT IMMUNITY TO RF WIRELESS COMMUNICATIONS EQUIPMENT Test Maximum MMUNITY Band Service Modulation Distance frequency power TEST LEVEL (MHz) (MHz) (V/m) Pulse modulation 380 –390 TETRA 400 18 Hz GMRS 460, ± 5 kHz deviation 430 – 470 FRS 460 1 kHz sine Pulse LTE Band 13,... -
Page 30: Input Ac Power Port
INPUT AC POWER PORT MMUNITY TEST LEVELS Basic EMC Phenomenon standard Professional healthcare facility environment Electrical fast transients / IEC 61000-4-4 ± 2 kV a) l) o) bursts 100 kHz repetition frequency a) b) j) o) IEC 61000-4-5 ± 0,5 kV, ± 1 kV Surges Line-to-line a) b) j) k) o) - Page 31 CONTINUED that do not have a surge protection device in the primary power circuit may E EQUIPMENT ME SYSTEMS be tested only at ± 2 kV line(s) to earth and ± 1 kV line(s) to line(s). Not applicable to C LASS II ME EQUIPMENT ME SYSTEMS Direct coupling shall be used.
-
Page 32: Patient Coupling Port
PATIENT COUPLING PORT MMUNITY TEST LEVELS Basic EMC Phenomenon standard Professional healthcare facility environment IEC 61000-4-2 ± 8 kV contact LECTROSTATIC DISCHARGE ± 2 kV, ± 4 kV, ± 8 kV, ± 15 kV air Conducted IEC 61000-4-6 disturbances 0,15 MHz – 80 MHz induced by RF fields in ISM bands between... -
Page 33: Signal Input/Output Parts Port
SIGNAL INPUT/OUTPUT PARTS PORT MMUNITY TEST LEVELS Basic EMC Phenomenon standard Professional healthcare facility environment IEC 61000-4-2 ± 8 kV contact LECTROSTATIC DISCHARGE ± 2 kV, ± 4 kV, ± 8 kV, ± 15 kV air Electrical fast IEC 61000-4-4 ±... -
Page 34: Recommended Separation Distances Between Portable And Mobile Rf Communications Equipment And Hausted Apc Series Models And Surgi-Chairs
RECOMMENDED SEPARATION DISTANCES BETWEEN PORTABLE AND MOBILE RF COMMUNICATIONS EQUIPMENT AND HAUSTED APC SERIES MODELS AND SURGI-CHAIRS The Hausted APC Series Models and Surgi-Chairs are intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the Hausted APC Series Models and Surgi-Chairs can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Hausted APC Series Models and Surgi-Chairs as recommended below, according to the maximum output power of the communications... -
Page 35: Index
10 INDEX Accessories, optional 19 Indications for Use 4 Accessories, optional, list 24 Info statement, significance 5 Advisory 4 IV rod, #18 (optional accessory), installing 19 APC / Surgi-Chair specifications 9 A word from GF Health Products, Inc. 4 Limited warranty 26 Low battery alarm 13 Back section, lowering 14 Lubrication 23... - Page 36 The most current and complete product information can be found on our website. www.grahamfield.com © 2014, GF Health Products, Inc. All Rights Reserved. Hausted is a trademark of GF Health Products, Inc., One Graham-Field Way, Atlanta GA 30340-3140 GF Health Products, Inc. is an ISO 13485 Certified Company.
















Need help?
Do you have a question about the HAUSTED EPC Series and is the answer not in the manual?
Questions and answers