Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Subscribe to Our Youtube Channel
Summary of Contents for Woodpecker LED.H
- Page 1 Guilin Woodpecker Medical Instrument Co., Ltd.
-
Page 2: Table Of Contents
Content 1 Principle and usage ...............1 2 Structure and component .............1 3 Technical specifications ............2 4 Installment and dismantlement ...........3 5 Operation ................3 6. Cleaning, Disinfection and Sterilization ......5 7 Cautions ................14 8 Contraindication ..............15 9 Maintenance ................15 10 Troubleshooting ..............16 11 After service ...............17 12 Storage and transportation ..........18 13 Environmental protection ..........18... -
Page 3: Principle And Usage
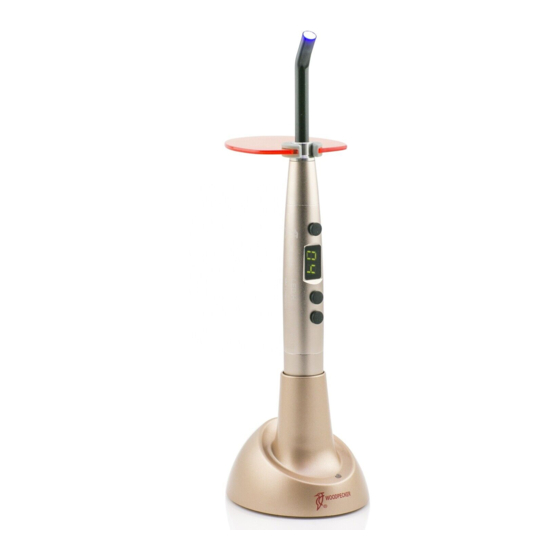
1 Principle and usage 1.1 LED.H adopts the principle of ray radiation to solidify the light- sensitive resin by shooting at it in a short time. 1.2 This product is used to restore teeth. 2 Structure and component LED.H (dental) is composed mainly of high power LED, optical fiber and main unit. -
Page 4: Technical Specifications
Figure 1 -Ⅱ 3 Technical specifications 3.1 Power supply: rechargeable Lithium battery Battery model: ICR18650 Battery voltage and capacity: 3.7V/2200mAh Input of adapter: AC100V to 240V, 50Hz/60Hz Output of adapter: DC5V/1A 3.2 Applied part: optical fiber 3.3 Light source: a) 5W high power blue LED b) Wave length: 385nm-515nm c) Light intensity: 1000mW/cm ~1200mW/cm... -
Page 5: Installment And Dismantlement
4.1 Take off the red cap from the optical fiber and then insert the metal part into the front of LED.H, make sure to screw the fiber to the end. 4.2 To install the light hood on the top of the optical fiber. - Page 6 5.1.1 Full power mode: screen shows 01, LED works in full power. 5.1.2 Ramping mode: screen shows 02, LED turns from weak to stronger, and reaches the highest power in 5 seconds. 5.1.3 Pulse mode: screen shows 03, LED works in the mode of pulse. 5.2 Lightly press the time button to choose the solidification time.
-
Page 7: Cleaning, Disinfection And Sterilization
affect the light intensity. 5.8 This equipment will turn off automatically if no any action within 2 minutes, turn it on by press any button. 5.9 The depth of solidification of composite is no less than 4mm per 10 seconds. 5.10 The optical fiber should be sterilized for 4 minutes with 134 ℃... - Page 8 The pro ducts have been designed for a large number of sterilization cycles. The materials used in manufacture were selected accordingly. However with every renewed preparation for use, thermal and chemical stresses will result in ageing of the products. The maximum number of sterilizations for optical fiber is 500 times.
- Page 9 2. Dry the product with a clean, soft cloth and place it in a clean tray. Notes a) The water used here must be pure water, distilled water or deionized water. 6.2 Preparation before cleaning Steps Tools: tray, soft brush, clean and dry soft cloth Remove optical fiber from main unit and put it into the clean tray.
- Page 10 It is recommended to use a washer-disinfector in accordance with EN ISO15883. For the specific procedure, please refer to the automated disinfection section in the next section “Disinfection”. Notes a) The cleaning agent does not have to be pure water. It can be distilled water, deionized water or multi-enzyme.
- Page 11 product is needed only when the product is removable in the device. The products are not allowed to contact each other. 2. Use a suitable rinsing adaptor, and connect the internal water lines to the rinsing connection of the washer-disinfector. 3.
- Page 12 of cleaner, the concentration and time provided by manufacturer shall be obeyed. The used cleaner is neodisher MediZym (Dr. Weigert). d) Disinfection: (d1) Direct use after disinfection: temperature ≥ 90 ° C, time ≥ 5 min or A0 ≥ 3000. (d2)Sterilize it after disinfection and use: temperature ≥...
- Page 13 completed. 2. It can also be dried directly in a medical drying cabinet (or oven). The recommended drying temperature is 80℃~120℃ and the time should be 15~40 minutes. Notes a) The drying of product must be performed in a clean place. b) The drying temperature should not exceed 138 °C;...
- Page 14 medical sterilization bag (or special holder, sterile box). Notes a) The package used conforms to ISO 11607; b) It can withstand high temperature of 138 °C and has sufficient steam permeability; c) The packaging environment and related tools must be cleaned regularly to ensure cleanliness and prevent the introduction of contaminants;...
- Page 15 b) Before using the sterilizer for sterilization, read the Instruction Manual provided by the equipment manufacturer and follow the instructions. c) Do not use hot air sterilization and radiation sterilization as this may result in damage to the product; d) Please use the recommended sterilization procedures for sterilization. It is not recommended to sterilize with other sterilization procedures such as ethylene oxide, formaldehyde and low temperature plasma sterilization.
-
Page 16: Cautions
a) The storage environment should be clean and must be disinfected regularly; b) Product storage must be batched and marked and recorded. 6.10 Transportation 1. Prevent excessive shock and vibration during transportation, and handle with care; 2. It should not be mixed with dangerous goodsduring transportation. 3. -
Page 17: Contraindication
7.5 It is forbidden to touch the charging connector with metal or other conductor, to avoiding damage the circuit of charge or the battery. 7.6 Please recharge the battery in cool and ventilated room. 7.7 It is forbidden to self-taking-apart the battery, in order not to result in short-circuit or leakage. -
Page 18: Troubleshooting
temperature and high pressure, other parts should be cleaned by clean water or neutral sterilized liquid, but do not soak the equipment in the water. Do not clean by volatile or soluble liquid, otherwise the marks of the control panel will fade. 9.3 Please clean the optical fiber to avoid the remaining resin on the surface and infect the life-span and the effectiveness of solidification. -
Page 19: After Service
Faulty Possible cause Solutions Light intensity is 1. The optical fiber is 1. Reinstall the optical weak. not installed well. fiber. 2. There is crevice on 2. Change a new optical the optical fiber. fiber. 3. There is resin on the 3. -
Page 20: Storage And Transportation
please refer to the warranty card for the warranty period. 12 Storage and transportation 12.1 This equipment should be handled carefully, kept away from shaking point, installed or stored at shadowy, dry, cool and ventilated places. 12.2 Don't store it together with articles that are combustible, poisonous, caustic and explosive. -
Page 21: Symbol Instructions
15 Symbol instructions Trademark CE marked product Type B applied part Screw inside/ outside Ordinary equipment Class II equipment Date of manufacture Manufacturer Recovery Used indoor only Keep dry Handle with care Sterilizable up to the temperature specified Temperature limitation for storage Humidity limitation for storage Atmospheric pressure for storage... -
Page 22: Statement
The pictures are only for reference. The final interpretation rights belong to GUILIN WOODPECKER MEDICAL INSTRUMENT CO., LTD. The industrial design, inner structure, etc, have claimed for several patents by WOODPECKER, any copy or fake product must take legal responsibilities. 17 EMC - Declaration of conformity The device has been tested and homologated in accordance with EN 60601-1-2 for EMC. - Page 25 ZMN-SM-157 V3.9-20200318...















Need help?
Do you have a question about the LED.H and is the answer not in the manual?
Questions and answers