Dräger Infinity M300 Supplement Manual
Hide thumbs
Also See for Infinity M300:
- Supplemental manual (46 pages) ,
- Instructions for use manual (318 pages)
Table of Contents

Summary of Contents for Dräger Infinity M300
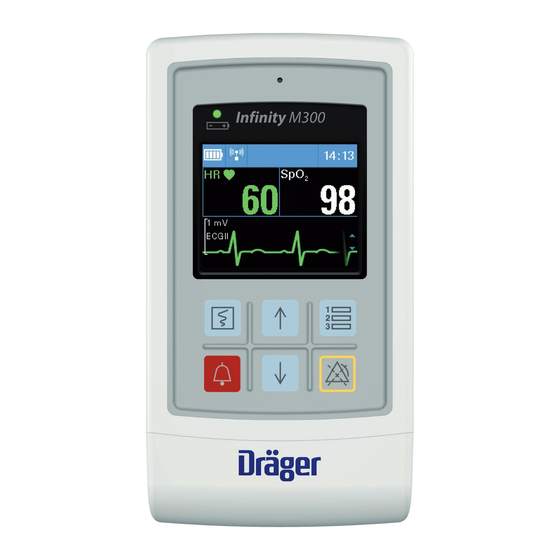
- Page 1 Supplement Infinity M300 ® Infinity ® M300 WARNING For a full understanding of the Software VG2.4 performance characteristics of this device, the user should carefully read this supplement and the related instructions for use before use of the device.
-
Page 3: Table Of Contents
Known issues ......44 Infinity M300 VG2.4..... - Page 4 This page has been left blank intentionally. Supplement – Infinity ® M300 – VG2.4...
-
Page 5: Information About This Document
Information about this document Information about this document Typographical conventions Consecutive numbers indicate steps of action, with the numbering restarting with "1" for each new sequence of actions. Bullet points indicate individual actions or different options for action. – Dashes indicate the listing of data, options, or objects. - Page 6 Information about this document Trademarks owned by third-party manufacturers Trademark Trademark owner rainbow ® Masimo Corporation. Masimo ® Masimo ® ® (Signal Extraction Technology) Capnoline Medtronic ® Microstream ® TOFscan ® IDMED Definitions WARNING A WARNING statement provides important information about a potentially hazardous situation which, if not avoided, could result in death or serious injury.
-
Page 7: Infinity M300 Vg2.4
Infinity M300 VG2.4 This supplement contains the VG2.4 instructions for use for the Infinity M300. The IFU that ships with this supplement is the Infinity CentralStation Wide SW VG 1.n and Infinity M300. Infinity M300 Device description The Infinity M300 is a wireless telemetry, patient-worn device with rechargeable lithium-ion battery which monitors ECG and SpO2 physiological data, features a color display, and local alarm alerts and keypad interface. - Page 8 To facilitate patient mobility the M300 can be placed in a disposable or re-usable shower pouch and worn by the patient. When a patient is sedentary (in bed or sitting) the clinician can place the Infinity M300 in the Bedside Charger to provide a slow charge for the device. When the Infinity M300 is not in clinical use, it may be stored and recharged at an accelerated rate in the CentralCharger.
-
Page 9: Indications For Use/Intended Use
Indications for use/Intended use Indications for use/Intended use The Infinity M300 is intended for use with the ICS to monitor ECG and pulse oximetry on ambulatory and non-ambulatory adult and pediatric patients using wireless communication over the Infinity patient monitoring network. -
Page 10: For Your Safety And That Of Your Patients
For your safety and that of your patients For your safety and that of your patients Mandatory reporting of adverse events Serious adverse events with this product must be reported to Dräger and the responsible authorities. General safety information The following WARNING and CAUTION statements apply to general operation of the medical device. Warnings: WARNING To properly use the M300 medical device, read and comply with the latest instructions for use... -
Page 11: Environment Of Use
Do not autoclave accessories. Environment of Use The Infinity M300 is intended to be used in an environment where patient care is provided by Healthcare Professionals, i.e. physicians, nurses, and technicians, trained on the use of the device, who determine when use of the device is indicated, based upon their professional assessment of the patient's medical condition. -
Page 12: Patient Population
For your safety and that of your patients Patient Population The Infinity M300 is intended for use with ambulatory and non-ambulatory adult and pediatric patients, (one patient at a time) excluding infants and neonates, in environments where patient care is provided by healthcare professionals. - Page 13 For your safety and that of your patients Dräger recommends that the responsible organization install and operate Infinity monitoring devices on separate, isolated, VLANs to reduce risk from network security vulnerabilities. Use of QoS with M300 is required to ensure optimal network data transmission. Without these measures there is an increased risk that critical events may go undetected in cases of malicious attack, which could result in patient harm.
- Page 14 For your safety and that of your patients Application Protocol Transport Protocol Port Number Client/Server Infinity Recorder 6000 Client Infinity Recorder 6050 Client Infinity RemoteRecord 7100 Server Infinity LocalDischarge 9200 Client Infinity LLIP 18000 Client In the presence of multicast network traffic, ‘Network Config Error’ will be annunciated on the M300 display and will be captured in the error log.
- Page 15 For your safety and that of your patients Infinity Network Diagram Example Configuration M300 message The following message may display on the M300: Priority Message Description Action None Network config error Multicast traffic is detected Contact your hospital on the VLAN. biomedical department and IT department for support.
-
Page 16: Device Symbols
Caution: Federal law restricts this device to sale by or on the order of a physician. Rx only Refer to the IFU, Infinity CentralStation Wide SW VG 1.n and Infinity M300, that ships with this supplement for additional device symbols. Supplement – Infinity ®... -
Page 17: Infinity M300 Features For Vg2.4
Infinity M300 Features for VG2.4 Infinity M300 Features for VG2.4 This section contains the Infinity M300 features for the VG2.4 release. QRS Threshold Depending on the software versions running on the ICS and the M300 devices, the procedure you follow to set the QRS threshold on the M300 will differ: ... - Page 18 Infinity M300 Features for VG2.4 To set the QRS Threshold using earlier ICS and M300 versions For ICS 2.1 or earlier, or M300 VG2.3 or earlier, from the Main ICS screen: 1 Click System setup on the ICS. 2 Click Layouts.
-
Page 19: M300 Arrhythmia Processing
Infinity M300 Features for VG2.4 M300 Arrhythmia processing Arrhythmias are identified using an internal detection process. This process does the following: – Filters out ECG signal artifacts – Detects the beat pattern – Classifies the beat pattern – Detects the rhythm When arrhythmia analysis is enabled, multiple alarm conditions may occur simultaneously. -
Page 20: Ecg Signal Processing And Display
Infinity M300 Features for VG2.4 ECG signal processing and display The M300 calculates heart rates within a range of 15 beats to 300 beats per minute, using the R-R intervals of the last 10 seconds. This calculation excludes the two longest and two shortest R-R intervals. -
Page 21: Ecg Precautions
Infinity M300 Features for VG2.4 To maintain a clear signal, change electrodes every 24 to 48 hours or more often when the following occurs: – ECG signal degradation – Excessive patient perspiration – Skin irritation Consider the following when selecting electrode sites: –... -
Page 22: Ics Telemetry Defaults Screen
QRS/ARR processing QRS/ARR processing channels Adult QRS threshold Normal Pediatric QRS threshold Normal Refer to the IFU, Infinity CentralStation Wide SW VG 1.n and Infinity M300, for additional screen descriptions of the ICS interface. Supplement – Infinity ® M300 – VG2.4... -
Page 23: M300 Demographic Screens
Infinity M300 Features for VG2.4 M300 Demographic screens The Infinity M300 two Demographic screens display patient demographic information. All patient demographic information is received from the ICS. Use the Up/Down arrows to jump from screen to screen. Screen 1 Name... -
Page 24: Alarm Settings
Infinity M300 Features for VG2.4 Alarm settings Low battery alarm When the battery power level has reached approximately 30 minutes of remaining charge, a Low battery message is reported visually and acoustically at both the ICS and M300 to indicate that the battery is low. -
Page 25: Technical Specifications
2 To discharge the patient, use the up and down arrows on the keypad and select Discharge. 3 Press the Views key to confirm that the patient was discharged. Refer to the IFU, Infinity CentralStation Wide SW VG1.n and Infinity M300 for the steps to admit a patient using the M300. Technical specifications... - Page 26 230 x 525 x 190 mm (9.0 x 20.7 x 7.5 in) Weight 6.8 kg (15 lb) Cooling Convection Connections Up to ten (10) Infinity M300 devices Electrical specifications Input Voltage 100 to 240 VAC Input frequency (Hz) 50/60 Hz...
- Page 27 Infinity M300 Features for VG2.4 Infinity M300 Bedside Charger (MS18620) Infinity M300 Bedside Charger Physical specifications Size, H x W x D 45.7 x 162.5 x 99.1 mm (1.8 x 6.4 x 3.9 in) Weight 224.0 g (7.9 oz) Cooling...
- Page 28 Infinity M300 Features for VG2.4 Infinity M300 Bedside Charger (MS29558) Infinity M300 Bedside Charger Physical specifications Size, H x W x D 45.7 x 162.5 x 99.1 mm (1.8 x 6.4 x 3.9 in) Weight 375 g (13.2 oz) to 447 g (15.8 oz) depending on plug style...
-
Page 29: M300 Performance Data
Infinity M300 Features for VG2.4 M300 Performance data The following disclosures related to algorithm performance using certain waveforms are added or updated to the monitoring specifications: M300 ECG/Arrhythmia/ST Supplemental information Time to alarm for Ventricular tachycardia 1mVpp, 206 bpm tachycardia Gain 0.5, range 3.0 to 3.5 seconds, average 3.3 seconds... -
Page 30: M300 Accessories Overview
M300 accessories overview M300 accessories overview Pulse Oximetry (SpO ) monitoring with Masimo (for M300) The following components are for the M300. Contact your Dräger representative for additional information. NOTE – For Masimo sensors, there are unique part numbers for Global, USA, and Japanese location references. - Page 31 M300 accessories overview M-LNCS Adapter Cables Global Part USA Part Japanese Description Number Number Part Number MP02994 MP03028 MP03107 Adapter Cable M-LNCS to LNC M-LNCS Adhesive SpO Sensors Global Part USA Part Japanese Description Number Number Part Number MP02976 MP03071 MP03173 Masimo M-LNCS Adtx-3, 20p MP02977...
- Page 32 M300 accessories overview Masimo RD-SET Adapters Global Part USA Part Japanese Description Number Number Part Number MS33731 MS33750 MS33769 RD to LNOP adapter MS33732 MS33751 MS33770 RD to LNCS adapter MS33734 MS33753 MS33772 RD to M-LNCs adapter RD-SET intermediate cables MS34436 intermediate cable Masimo RD-SET, 1.2 m MS34437...
-
Page 33: Electromagnetic Compatibility (Emc)
Electromagnetic compatibility (EMC) EMC third edition The Infinity M300 device (MS25755) revisions 19 and below, which are hardware revision 3, meet EMC 3rd edition requirements as described in this section. The Infinity M300 has been designed and tested for compliance with current regulatory standards as to its capacity to limit electromagnetic emissions (EMI), and also as to its ability to block the effects of EMI from external sources. - Page 34 ‘quieter’ the electrical environment the better. In general, increasing the distance between electrical devices decreases the likelihood of interference. NOTE The Infinity CentralStation and Infinity M300 are intended for use in the electromagnetic environments specified below. The user of this equipment should assure that is used in an environment meeting these specifications.
- Page 35 Electromagnetic compatibility (EMC) Electromagnetic immunity Immunity IEC 60601-1-2 Compliance Electromagnetic environ- against... 3rd edition level (of this ment test level device) ±6 kV Electrostatic Contact discharge: Floors should be wood, con- ±6 kV discharge, ESD crete or ceramic tile. If floors (IEC 61000-4-2) are covered with synthetic ma- ±8 kV...
- Page 36 Electromagnetic compatibility (EMC) Electromagnetic immunity Immunity IEC 60601-1-2 Compliance Electromagnetic environ- against... 3rd edition level (of this ment test level device) Conducted RF 150 kHz to 80 MHz: 3 Vrms Portable and mobile RF com- munications equipment should RF coupled into be used no closer to any part of lines the monitor, including cables,...
- Page 37 Electromagnetic compatibility (EMC) Electromagnetic immunity Immunity IEC 60601-1-2 Compliance Electromagnetic environ- against... 3rd edition level (of this ment test level device) Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast, and TV broadcast cannot be predicted theoretically with accuracy.
-
Page 38: Emc Fourth Edition
Specification) and by ETSI, IEC 60601-1-2, will not be exceeded. These limits are part of interna- tional safety standards and defined by international committees. NOTE The Infinity M300 complies with part 15 of the FCC rules. Operation is subject to the following two con- ditions: – This device may not cause harmful interference. - Page 39 The instructions for use for the other devices must be followed. Electromagnetic environment This device may only be used in environments specified in section "Infinity M300 Device description" on page 7. Emissions Compliance...
- Page 40 Electromagnetic compatibility (EMC) Immunity against Test level and required electromagnetic IEC 60601-1-2 4th edition environment Electrostatic discharge (ESD) (IEC 61000-4-2) Contact discharge: ±8 kV Air discharge: ±15 kV Magnetic fields at mains frequency 30 A/m (IEC 61000-4-8) Radiated high-frequency disturbances 80 MHz to 2.7 GHz: 3 V/m (IEC 61000-4-3) Conducted high-frequency disturbances...
-
Page 41: Additional Information
Additional information Additional information ECG, arrhythmia, and ST segment monitoring with M300 The ST algorithm has been tested for accuracy of the ST segment data. The significance of the ST segment changes need to be determined by a clinician. NOTE ST analysis is always performed using a dedicated filter which ensures diagnostic quality. - Page 42 Additional information Pacemaker precautions WARNING Risk of pacer-induced artifacts on M300 waveform The M300 has been tested for pacemaker pulse detection. However, it is impossible to anticipate every clinically possible waveform characteristic. For paced patients, the M300 could therefore miscount heart rates and misinterpret rate-dependent arrhythmias.
- Page 43 Additional information WARNING Always keep pacemaker patients under close surveillance and monitor their vital signs carefully. – Do not assess the patient’s condition exclusively from the heart rate values the monitor dis- plays and the rate alarms that are generated. Heart rate meters may continue to count the pacemaker rate during cardiac arrest or some arrhythmias.
-
Page 44: Known Issues
False pacer spikes may affect the accuracy of ECG beat classification. To resolve this issue: – Be sure to use new electrodes. – Temporarily remove the Infinity M300 from the bedside charger until the electrode impedance normalizes. – Turn pacer detection off, if appropriate. -
Page 45: Reprocessing
Reprocessing Reprocessing This section provides information for the reprocessing of M300 device-specific components and accessories. Keep this supplement with the instructions for use. Safety Information WARNING Risk due to inappropriately reprocessed products Reusable products must be reprocessed, otherwise there is an increased risk of infection. –... -
Page 46: Classification For Reprocessing
Reprocessing Classification for reprocessing Classification of medical devices The classification depends on the intended use of the medical device. The risk of infection transmission through the application of the product to the patient without proper reprocessing is the basis of the Spaulding classification. -
Page 47: Before Reprocessing
Reprocessing Before reprocessing Observe before disassembly WARNING A risk of an electric shock might occur due to penetrating liquid. Follow the instructions below to disconnect power before reprocessing. 1 Turn off the M300 Telemetry Device: a. Simultaneously press and hold both arrow keys. b. -
Page 48: Validated Reprocessing Procedures
Reprocessing Removing the accessories – ECG lead wire – SpO2 intermediate cable – Pouch M300 components Validated reprocessing procedures Overview of the reprocessing procedures of the components Components Surface disin- Manual clean- Machine Steam Description of the fection with ing followed cleaning with steriliza- procedure... -
Page 49: Cleaning
Reprocessing WARNING Risk of electric shock and device malfunction. Penetrating liquid may cause the following: – Damage to the device – Electric shock – Device malfunctions Ensure that no liquid penetrates the device. WARNING Do not immerse or rinse the device and its peripherals. If you spill liquid on the device (including the battery or accessories), or accidentally immerse it in liquid, allow contacts to thoroughly dry. -
Page 50: Surface Disinfection
Reprocessing Surface disinfection 4 Take a new disposable cloth soaked in surface disinfectant. Wipe cleaned surfaces again until all surfaces to be disinfected are visibly wet. 5 Wait 15 minutes for the surface disinfectant contact time. 6 At the end of the contact time, moisten a new, uncontaminated and lint-free cloth with water (at least drinking water quality). -
Page 51: Other Agents And Reprocessing Procedures
Reprocessing Other agents and reprocessing procedures Disinfectants Use nationally approved disinfectants suitable for the respective reprocessing process and the intended application. Surface disinfectants The manufacturers of the surface disinfectants have verified at least the following spectra of activity: – Bactericidal –... -
Page 52: Reprocessing Of Patient-Specific Accessories
Reprocessing Reprocessing of patient-specific accessories Categorization of accessories Category Classification Part number Description of the procedure ECG monolead Non-Critical MS14555 See Surface disinfection with MS14556 cleaning on pages MS14559 57 - 59. MS14560 MS14683 MS14682 SpO2 intermediate cable Non-Critical MS18683 MS17330 SpO2 sensor Non-Critical... -
Page 53: Cleaning
Reprocessing WARNING Risk of electric shock and device malfunction. Penetrating liquid may cause the following: – Damage to the device – Electric shock – Device malfunctions Ensure that no liquid penetrates the device. CAUTION To avoid damaging the device, do not use sharp tools or abrasives. Never immerse electrical connectors in water or other liquids. -
Page 54: Surface Disinfection
Reprocessing Surface disinfection 4 Take a new disposable cloth soaked in surface disinfectant. Wipe cleaned surfaces again until all surfaces to be disinfected are visibly wet. 5 Wait 15 minutes for the surface disinfectant contact time. 6 At the end of the contact time, moisten a new, uncontaminated and lint-free cloth with water (at least drinking water quality). -
Page 55: Other Agents And Reprocessing Procedures
Reprocessing Other agents and reprocessing procedures Disinfectants Use nationally approved disinfectants suitable for the respective reprocessing process and the intended application. Surface disinfectants The manufacturers of the surface disinfectants have verified at least the following spectra of activity: – Bactericidal –... -
Page 56: After Reprocessing
– The device has been assembled and prepared so that it is ready for operation. Check the operational readiness (see the instructions for use, chapter “Getting started“in the IFU, Infinity CentralStation Wide SW VG 1.n and Infinity M300). Supplement – Infinity ®... - Page 58 This document is provided for customer information only, and will not be updated or exchanged without customer request. The radio equipment in the Infinity M300 patient monitor complies with the Radio Equipment Directive (2014/53/EU). A copy of the Declaration of Conformity is available at the following Internet address: www.draeger.com/doc-radio...











Need help?
Do you have a question about the Infinity M300 and is the answer not in the manual?
Questions and answers