Summary of Contents for Covidien Nellcor Series
- Page 1 Operator’s Manual Addendum Nellcor™ Bedside Respiratory Patient Monitoring System Respiration Rate Version 1.0...
- Page 2 Covidien’s sole responsibility with respect to the equipment and software, and its use, is as stated in the limited warranty provided. Nothing in this manual shall limit or restrict in any way Covidien’s right to revise or otherwise change or modify the equipment and software described herein, without notice. In the...
-
Page 3: Table Of Contents
Table of Contents Overview ........1 Introduction . -
Page 5: Overview
For first time activation of the parameter, review the activation kit instructions or contact Covidien for more details. Use this addendum after thoroughly reading the Operator’s Manual. All instruc- tions, other than those listed here, remain the same as those listed in the Opera- tor’s Manual. - Page 6 Nellcor™ Respiration Rate Table 1-1. Additions to the Operator’s Manual Chapter Title Respiration Rate Addition Introduction Additional warnings and cautions Product Overview Additional product description and intended use statements, New monitoring screen fields included in Operation section. Installation Refer to separate Activation Instructions Operation Additional monitoring screen views, additional alarm condi- tions, additional sensor type...
-
Page 7: Introduction
It must be used in conjunction with clinical signs and symptoms. WARNING: Use only Covidien-approved sensors and interface cables when connecting to the sensor port. Connecting any other cable or sensor influences the accuracy of sensor data, which may lead to adverse results. - Page 8 Nellcor™ Respiration Rate Caution: Accuracy of Respiration Rate was established using bench-top testing and clinical studies in 26 healthy volunteers and 53 hospitalized patients. Hospital studies were conducted using convenience sampling and did not necessarily include all patient conditions found in hospitals and hospital-type settings. These clinical study results may not generalize to all patient conditions.
-
Page 9: Related Documents
Pulse Oximetry Sensor Instructions for Use — Guides sensor selection and • usage. Before attaching any of the various Covidien-approved Nellcor™ sensors to the monitoring system, refer to their Instructions for Use. Saturation Accuracy Grid — Provides sensor-specific guidance related to •... -
Page 10: Warranty Information
Nellcor™ Respiration Rate Warranty Information 1.2.3 To obtain information, if any, contact Covidien or a local Covidien representative. Covidien Technical Services: Patient Monitoring 15 Hampshire Street Mansfield, MA 02048 USA 1.800.635.5267, 1.925.463.4635 (toll) or contact a local Covidien representative www.covidien.com... -
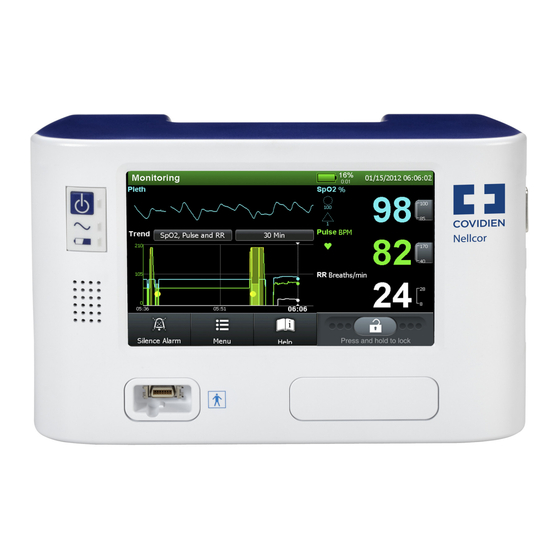
Page 11: Updated Monitoring Screen
Product Overview ed only for facility-use care of adults to detect patterns of desaturation indicative of repetitive reductions in airflow through the upper airway and into the lungs. The Respiration Rate parameter, when used in conjunction with the Nellcor™ Bedside Respiratory Patient Monitoring System and Nellcor™ Respiratory Sensor is intended for the continuous, non-invasive monitoring of respiration rate in adult patients in hospitals and hospital-type facilities. -
Page 12: Operation
Nellcor™ Respiration Rate Operation Required Pulse Oximetry Sensor Usage 1.4.1 To obtain respiration rate, caregivers must use a Nellcor™ Respiratory Sensor. If caregivers use alternate sensors, listed in the Operator’s Manual, the moni- toring system continues to post both SpO and pulse rate data, with the user message, NON-RR SENSOR CONNECTED, and dashes in the RR field. -
Page 13: Prerequisites
Operation on the horizontal axis. The trend view shows the patient’s respiration rate over time, with the most current reading on the right. Prerequisites 1.4.2 Before starting a monitoring session, confirm the following: The monitoring system is powered on, has successfully completed its power-on •... -
Page 14: Respiration Rate Views
Nellcor™ Respiration Rate Respiration Rate Views 1.4.3 Figure 1-5. Pleth View with Respiration Rate Figure 1-6. Trend View with Respiration Rate ADD-10 Operator’s Manual Addendum... -
Page 15: Additional Alarm Messages
Operation Figure 1-7. Combined View with Respiration Rate Figure 1-8. Blip View with Respiration Rate Additional Alarm Messages 1.4.4 Existing alarm behaviors remain the same as those listed in the Operator’s Manual. Several new alarms accompany the respiration rate parameter. When an alarm is active, the value in that field turns black on a yellow background. -
Page 16: Additional Respiration Rate Parameter
Nellcor™ Respiration Rate Figure 1-9. Sample Alarm Condition Additional Respiration Rate Parameter 1.4.5 WARNING: Use only Nellcor-approved sensors and interface cables when connecting to the sensor port. Connecting any other cable or sensor influences the accuracy of sensor data, which may lead to adverse results. ... -
Page 17: Menu Options And Defaults
Operation cant changes in respiration rate over time may indicate that patient status is deteriorating and additional assessment is warranted. Reference the Reference Theory of Operations, p. ADD-24,for the theory behind how the Respiration Rate parameter works. Reference Monitoring System Constraints, p. - Page 18 Nellcor™ Respiration Rate The trend data pop-up window also includes respiration rate data whenever the Respiration Rate parameter is enabled. Figure 1-11. MONITORING HISTORY Pop-up Trend data types in the SELECT TRENDS option also include variations of the three main variables of SpO , pulse, and respiration rate.
-
Page 19: Additional Data Output Fields
Additional Data Output Fields Figure 1-12. Initial Select Trends Screen Additional Data Output Fields The following trend data fields are available to users after activating respiration rate. Not all remote monitoring software will report respiration rate data. (Saturation) trend data • Pulse rate (BPM) trend data •... - Page 20 Nellcor™ Respiration Rate Figure 1-13. Graphical trend data monitoring history components Graph x-axis contains time divisions, depending on specified time scale Yellow triangle icon for period of SPD alert Green pulse trend data for specified time scale Graph y-axis contains signal amplitude values Trend scale button for adjusting time scale to view more or less data Dashed horizontal yellow lines for upper and lower thresholds Yellow circle icon for period of SatSeconds alarm...
-
Page 21: Historical Trend Data
Additional Data Output Fields Figure 1-14. Tabular trend data components Column heading(s) Time and date stamp Yellow highlight, indicating an upper or lower limit threshold violation Trend scale button for adjusting time scale to view more or less data Status message, indicating an alarm condition Red highlight, indicating a high priority alarm Respiratory trend data (Zeroes indicate missing trend data) Historical Trend Data... - Page 22 Nellcor™ Respiration Rate Figure 1-15. Historical Trend Data with Respiration Rate Values Time stamp Alarm type High and low readings Pulsatile strength minimum/maximum Upper and lower limits Sample interval (period in seconds) Respiration rate data ADD-18 Operator’s Manual Addendum...
-
Page 23: Performance Considerations
Inspect the sensor site as directed in the Instructions for Use. WARNING: Use only Covidien-approved sensors and interface cables when connecting to the sensor port. Connecting any other cable or sensor influences the accuracy of sensor data, which may lead to adverse results. - Page 24 Nellcor™ Respiration Rate Caution: Accuracy of respiration rate was established using bench-top testing and clinical studies in 26 healthy volunteers and 53 hospitalized patients. Hospital studies were conducted using convenience sampling and did not necessarily include all patient conditions found in hospitals and hospital-type settings. These clinical study results may not generalize to all patient conditions.
- Page 25 Performance Considerations Inaccurate Sensor Measurement Conditions A variety of conditions can cause inaccurate sensor measurements. Incorrect application of the recommended sensor • Placement of the recommended sensor on an extremity with a blood pressure cuff, • arterial catheter, or intravascular line Ambient light •...
-
Page 26: Patient Conditions
Nellcor™ Respiration Rate direct sunlight can interfere with sensor performance. To prevent interference from ambient light, ensure the recommended sensor is properly applied and cover the sensor with opaque material. If patient activity presents a problem, try one or more of the following remedies to correct the problem. -
Page 27: Troubleshooting
Troubleshooting Excessive patient talking – Respiration rate outside the range of 4 to 40 breaths per minute – Significantly irregular cardiac rhythms (three or more events of irregularity – observed within 30 seconds) Troubleshooting Existing troubleshooting and error codes remain the same as those listed in the Operator’s Manual. -
Page 28: Additional Nellcor™ Pulse Oximetry Sensor
Use the recommended sensor’s Instructions for Use to guide sensor selection or contact Covidien or a local Covidien representative. Reference Nellcor™... - Page 29 Theory of Operations In clinical settings, both the cardiac pulse and baseline components may vary over time due to physiologic conditions and changes. In standard pulse oxim- etry, these variations are typically filtered out in order to accurately measure arterial oxygen saturation (SpO ).
-
Page 30: Specifications
(ARMS) range. For a complete listing and for test results of SpO accuracy across the full line of available Nellcor™ sensors, contact Covidien, a local Covidien representative, or locate it online at www.covidien.com. ADD-26... -
Page 31: Clinical Studies
Clinical Studies Clinical Studies 1.11 Two (2) prospective, observational, non-randomized clinical studies were conduct- ed to demonstrate the accuracy of the Respiration Rate Version 1.0 when used in conjunction with the Nellcor™ Bedside Respiratory Patient Monitoring System, using the Nellcor™ Respiratory Sensor. One study was performed with healthy vol- unteers at a single clinical laboratory. - Page 32 Nellcor™ Respiration Rate Table 1-7. Medical Conditions in Hospitalized Patients Cardiovascular Renal (continued) Hypertension Nephrolithiasis Deep Venous Thrombosis (DVT) Amyloidosis Coronary Artery Disease Hyperphosphatemia Congestive Heart Failure Metabolic Acidosis Previous Myocardial Infarction Adrenal Neoplasm Fluid Overload and/or Venous Insufficiency Genitourinary Asymptomatic Bradycardia Urinary Tract Infection Diastolic Dysfunction Gynecologic Tumor...
-
Page 33: Study Results
Clinical Studies Study Results 1.11.3 Results for Respiration Rate Version 1.0 are shown in Table 1-8. The observed respiration rate range in the studies was 4 to 34 breaths per minute. Accuracy was calculated using both root mean square difference (RMSD) and mean error. -
Page 34: Adverse Events
Nellcor™ Respiration Rate Figure 1-17. Modified Bland-Altman Plot of Nellcor Respiration Rate Software versus Reference (N=23,243 Paired Observations) Difference (Reference—Nellcor Mean + (1.96 x sd) Respiration Rate Software) Mean Reference Mean - (1.96 x sd) Adverse Events 1.11.4 No adverse events were reported during the studies. Conclusion 1.11.5 In clinical studies of healthy subjects and hospitalized patients, Respiration Rate,... - Page 36 Part No. 10084254 Rev D 2013-10 © 2013 Covidien. Covidien Ireland Limited, IDA Business and Technology Park,Tullamore. Covidien llc 1.800.255.6774 [T] www.covidien.com 15 Hampshire Street Mansfield, MA 02048...













Need help?
Do you have a question about the Nellcor Series and is the answer not in the manual?
Questions and answers