KaVo EXPERTsurg LUX Instructions For Use Manual
Hide thumbs
Also See for EXPERTsurg LUX:
- Instructions for use manual (66 pages) ,
- Instructions for use manual (68 pages) ,
- Technician's instructions (54 pages)
Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for KaVo EXPERTsurg LUX
- Page 1 Instructions for use EXPERTsurg LUX – 1.008.3500...
- Page 2 Manufacturer: Distributed by: KaVo Dental Technologies, LLC KaVo Dental GmbH 11727 Fruehauf Drive Bismarckring 39 Charlotte, NC 28273 USA 88400 Biberach Germany Phone: 847 550 6800 Fax: 847 550 6825 www.kavo.com...
-
Page 3: Table Of Contents
Instructions for use EXPERTsurg LUX – 1.008.3500 Table of contents Table of contents 1 User instructions ....................... 2 Safety..........................2.1 Explosion hazard ......................7 2.2 Infection hazard ......................7 2.3 Electrical shock......................7 2.4 Technical condition ......................8 2.5 Electromagnetic fields ....................8 2.6 Improper use ........................ - Page 4 Instructions for use EXPERTsurg LUX – 1.008.3500 Table of contents 5.6.1 Manual rinsing function ..................43 5.6.2 Program step Rinsing function ................44 5.7 Activating the one-touch calibration................. 44 5.8 Foot control ........................46 5.8.1 Changing the speed, coolant flow rate, and direction of motor rotation....46 5.8.2...
-
Page 5: User Instructions
1 User instructions 1 User instructions Dear User, Congratulations on purchasing this KaVo quality product. By following the in- structions below you will be able to work smoothly, economically and safely. © Copyright by KaVo Dental GmbH KaVo and EXPERTsurg are either registered trademarks or trademarks of KaVo Dental GmbH. - Page 6 Please refer to the serial number of the product in all inquiries! For further infor- mation, please visit: www.kavo.com KaVo Repair Service For repairs, please contact the KaVo Repair Service. For scheduling or if you have any questions, please contact: KaVo Dental Technologies, LLC...
-
Page 7: Safety
Instructions for use EXPERTsurg LUX – 1.008.3500 2 Safety | 2.1 Explosion hazard 2 Safety NOTE All serious events occurring in relation to the product must be reported to the manufacturer and the competent authority of the member state, in which the user and/or patient resides. -
Page 8: Technical Condition
Instructions for use EXPERTsurg LUX – 1.008.3500 2 Safety | 2.4 Technical condition 2.4 Technical condition A damaged device or components can injure patients, users and third parties. 4 Use the device and components only if there is no damage on the outside. -
Page 9: Improper Use
Repairs and safety checks may only be performed by trained service personnel. The following persons are authorized to do this: ▪ Service technicians of KaVo branches after the appropriate product training ▪ Service technicians of KaVo authorized dealers after the appropriate product... -
Page 10: Information On Electromagnetic Compatibility
Instructions for use EXPERTsurg LUX – 1.008.3500 2 Safety | 2.10 Information on electromagnetic compatibility As a result of the use of NON-KaVo original spare parts during the repair, parts such as covers may become undone and injure the patient, user or other peo- ple. -
Page 11: Product Description
The tube set is needed to deliver the external coolant from the bottle to the different handpieces. A power cord provides elec- tric power to the unit. The EXPERTsurg LUX will be delivered with software on the surgical control unit. - Page 12 3 Product description | 3.1 Intended use ▪ avoid contamination from the product To guarantee the consistent readiness for use and to preserve the value of the KaVo product, the recommended maintenance services must be carried out in 2 year intervals. NOTE The permitted work is described in the Technician's Instructions available to the trained service staff.
-
Page 13: Scope Of Delivery
Instructions for use EXPERTsurg LUX – 1.008.3500 3 Product description | 3.2 Scope of delivery 3.2 Scope of delivery The scope of delivery of the EXPERTsurg LUX includes the following: ▪ Device EXPERTsurg LUX ▪ Foot control ▪ Surgical motor INTRA LUX S600 LED ▪... -
Page 14: Expertsurg Lux
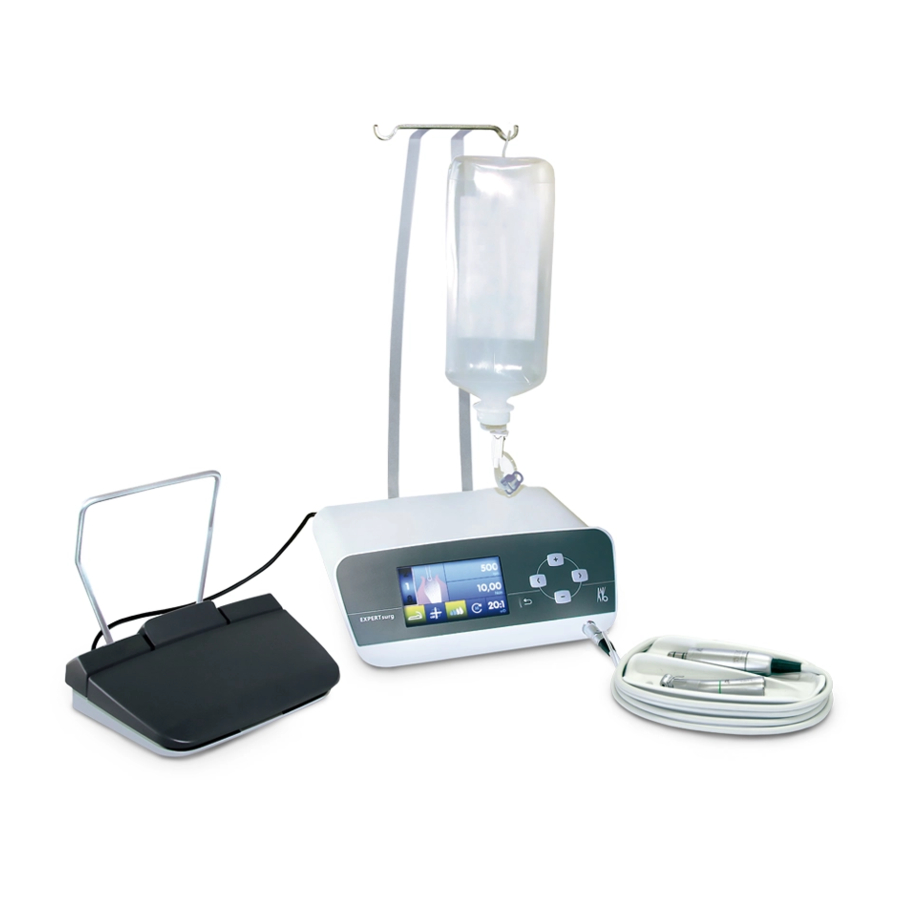
Instructions for use EXPERTsurg LUX – 1.008.3500 3 Product description | 3.3 EXPERTsurg LUX 3.3 EXPERTsurg LUX Product reference guide, front view ① Hand-held control panel ⑥ Surgical motor ② Bottle holder ⑦ Coolant hose ③ Hose pump ⑧ Handpiece holder ④... - Page 15 Instructions for use EXPERTsurg LUX – 1.008.3500 3 Product description | 3.3 EXPERTsurg LUX Rear Product reference guide, rear view ① Hose pump locking mechanism ⑤ Follow instructions for use ② On-button ⑥ SD card slot ③ Mains plug ⑦ Foot control electrical outlet ④...
-
Page 16: Hand-Held Control Panel
Instructions for use EXPERTsurg LUX – 1.008.3500 3 Product description | 3.4 Hand-held control panel 3.4 Hand-held control panel ① Program step ⑩ Minus key, decrease value ② Display of the activity ⑪ Back key ③ Maximum torque reached ⑫ Transmission ratio ④... -
Page 17: Foot Control
Instructions for use EXPERTsurg LUX – 1.008.3500 3 Product description | 3.5 Foot control 3.5 Foot control ① Speed key (grey) ③ Program key (grey) ② Pump key (blue) ④ Direction of motor rotation key (yellow) 17 / 68... -
Page 18: Technical Specifications Expertsurg Lux
Instructions for use EXPERTsurg LUX – 1.008.3500 3 Product description | 3.6 Technical Specifications EXPERTsurg LUX 3.6 Technical Specifications EXPERTsurg LUX Width 265 mm Depth 255 mm Height 100 mm Weight Approx 1.9 kg Weight Foot control Approx 1.1 kg Weight motor Approx. 125 g Input voltage 100 –... -
Page 19: Symbols On Product, Packaging And Rating Plate
3 Product description | 3.7 Symbols on product, packaging and rating plate 3.7 Symbols on product, packaging and rating plate The product, packaging or rating plates bear the following symbols. The rating plates of EXPERTsurg LUX and foot control are both affixed on the underside of the housing. Accompanying documents... -
Page 20: Transport And Storage
Instructions for use EXPERTsurg LUX – 1.008.3500 3 Product description | 3.8 Transport and storage Supply voltage Protection class II Transportation and storage conditions (temperature range) Transportation and storage conditions (air pressure) Transportation and storage conditions (humidity) Protect from moisture... - Page 21 Freight Forwarders' Terms and Conditions, Art. 28). Outside Germany NOTE KaVo shall not be held liable for damage arising from transportation. The shipment must be checked on arrival. If the packaging is visibly damaged on delivery, please proceed as follows: 1.
-
Page 22: Startup
Instructions for use EXPERTsurg LUX – 1.008.3500 4 Startup | 4.1 Unpacking 4 Startup WARNING Hazard from contaminated products. Infection hazard for dentist and patient. 4 Prior to initial startup and after each use, process the product and acces- sories. -
Page 23: Connecting The Surgical Motor
Instructions for use EXPERTsurg LUX – 1.008.3500 4 Startup | 4.4 Connecting the surgical motor 4 Slide the bracket to the limit stop into the designated recesses. 4.4 Connecting the surgical motor NOTE The delivered parts are not sterile (except for the coolant hose). Before the first treatment of a patient, the surgical motor, motor cable, and handpiece holder need to be processed and sterilized. -
Page 24: Connecting The Coolant Container And Hose Set
Instructions for use EXPERTsurg LUX – 1.008.3500 4 Startup | 4.5 Connecting the coolant container and hose set ① Motor coupling ③ Handpiece holder ② Surgical motor ④ Plug of motor cable 4 Plug the surgical motor ② into the motor coupling ① and secure it with a union nut. - Page 25 Instructions for use EXPERTsurg LUX – 1.008.3500 4 Startup | 4.5 Connecting the coolant container and hose set NOTE The coolant must be selected to suit the planned application. The flow rate of the coolant is dependent on the instrument used. The user must set an ade- quate flow of coolant and check this.
- Page 26 Instructions for use EXPERTsurg LUX – 1.008.3500 4 Startup | 4.5 Connecting the coolant container and hose set ① Coolant hose ⑤ Pump hose ② Clips ⑥ Puncture needle ③ Hose fixation ⑦ Hose clamp ④ Lock 4 Close the hose clamp ⑦ of the hose set.
- Page 27 Instructions for use EXPERTsurg LUX – 1.008.3500 4 Startup | 4.5 Connecting the coolant container and hose set ca.10 mm 4 Open the lock ④ and insert the pump hose ⑤. 4 Close the lock ④. NOTE Make sure to place the pump hose in the pump appropriately such that the pump hose does not get clamped or pinched by the lock.
-
Page 28: Electrical Connection
Instructions for use EXPERTsurg LUX – 1.008.3500 4 Startup | 4.6 Electrical connection 4 Stick the puncture needle ⑥ into the coolant container and hook-in the coolant container on the bottle holder. 4 Check the sealing and firm seating of the puncture needle ⑥. Prevent fluid from leaking above the device. - Page 29 Instructions for use EXPERTsurg LUX – 1.008.3500 4 Startup | 4.6 Electrical connection NOTE The unit must be set-up appropriately such that the mains plug and the elec- trical outlet are easily accessible. NOTE The protective earth conductor is used as functional earthing (FE) rather than as protective earthing (PE).
-
Page 30: Operation
5.1 Turning the device on 4 Turn the device on. ð The device runs a self test. 4 KaVo recommends switching off the unit after use. NOTE Auto-Off function. After 10 minutes of inactivity, the light on the handpiece, the pump and the motor on the unit are turned off. - Page 31 Instructions for use EXPERTsurg LUX – 1.008.3500 5 Operation | 5.2 Making the device settings The following device settings need to be made once during the initial start-up of the device and can be adapted according to need. The software version in use can only be displayed, but not changed in the device settings.
-
Page 32: Surgical Motor Intra Lux S600 Led
Instructions for use EXPERTsurg LUX – 1.008.3500 5 Operation | 5.3 Surgical motor INTRA LUX S600 LED View Setting The LCD brightness determines the brightness of the display. The brightness can be set in 3 steps ranging from dark to maximum brightness. -
Page 33: Attaching The Straight Or Contra-Angle Handpiece
4 Pull on the KaVo handpiece to make sure that the KaVo handpiece is se- curely attached to the motor. -
Page 34: Defining And Running Program Steps
4 Twist the straight or contra-angle handpiece slightly to pull it off. 5.4 Defining and running program steps The EXPERTsurg LUX is based on program steps and associated activities and can be operated intuitively using the graphical user interface. Program step 1: Marking The current program step is shown on the display as a number ①... -
Page 35: Factory Settings
Instructions for use EXPERTsurg LUX – 1.008.3500 5 Operation | 5.4 Defining and running program steps 5.4.1 Factory settings The following program steps are pre-set at the factory: Program Symbol Activity Speed Torque Transmis- Coolant flow step [rpm] [Ncm] sion ratio... -
Page 36: Exemplary Program Step Sequences
Instructions for use EXPERTsurg LUX – 1.008.3500 5 Operation | 5.4 Defining and running program steps Program Symbol Activity Speed Torque Transmis- Coolant flow step [rpm] [Ncm] sion ratio rate Treatment – – – – completed (can be set from program... -
Page 37: Selecting The Program Steps
Instructions for use EXPERTsurg LUX – 1.008.3500 5 Operation | 5.4 Defining and running program steps Example 3: "Free application" activity as step 1, screw implant in manually Step Activity Free application Marking Pilot drilling Template Treatment com- drilling pleted (can be... -
Page 38: Selecting Activities
Instructions for use EXPERTsurg LUX – 1.008.3500 5 Operation | 5.4 Defining and running program steps 5.4.4 Selecting activities 4 Press the arrow keys until the activities display is shown. 4 Press the plus or minus keys to select the desired activity. -
Page 39: Changing Default Values
5.5.2 Setting the torque limit NOTE The EXPERTsurg LUX reduces the power to prevent the maximum torque set- ting from being exceeded. This may lead to the motor coming to a standstill if the rotating handpiece is blocked. There is an audible signal as soon as the maximum torque is reached. -
Page 40: Setting The Coolant Flow Rate
Tissue damage. 4 Please note the instructions for use of the attachment tool. 4 Set a sufficient coolant flow rate. The coolant flow rate of the KaVo surgical units can be set to 4 levels or switched off: Displayed informa-... -
Page 41: Changing The Direction Of Motor Rotation
Instructions for use EXPERTsurg LUX – 1.008.3500 5 Operation | 5.5 Changing default values 4 Press the arrow keys until the coolant display is highlighted. 4 Press the plus and minus keys to set the desired value. The step width de- pends on the transmission ratio and the range of values. -
Page 42: Setting The Transmission Ratio
Instructions for use EXPERTsurg LUX – 1.008.3500 5 Operation | 5.5 Changing default values 4 Press the plus and minus keys concurrently to change the direction of motor rotation. The direction of motor rotation can be changed during the treatment using the direction of motor rotation key of the foot control. -
Page 43: Rinsing Function
Instructions for use EXPERTsurg LUX – 1.008.3500 5 Operation | 5.6 Rinsing function 5.6 Rinsing function 5.6.1 Manual rinsing function The rinsing function serves to feed coolant and to start-up the illumination on the handpiece. The motor is not activated during this process. -
Page 44: Program Step Rinsing Function
NOTE The handpiece must be attached for calibration. One-touch calibration should be carried out only with KaVo surgical hand- pieces with a transmission ratio of 16:1, 20:1 or 27:1. The one-touch calibration cannot be run with third-party handpieces or hand- pieces with different transmission ratios. - Page 45 Instructions for use EXPERTsurg LUX – 1.008.3500 5 Operation | 5.7 Activating the one-touch calibration CAUTION The motor starts at full speed. Risk of injury. 4 Hold the motor firmly or put it in a safe holder during the calibration.
-
Page 46: Foot Control
Instructions for use EXPERTsurg LUX – 1.008.3500 5 Operation | 5.8 Foot control 5.8 Foot control 5.8.1 Changing the speed, coolant flow rate, and direction of motor rotation Control element Function 4 Press the foot pedal of the foot control in order to start the motor and increase the speed. -
Page 47: Changing The Coolant Container
Instructions for use EXPERTsurg LUX – 1.008.3500 5 Operation | 5.8 Foot control 5.8.3 Changing the coolant container The coolant container can be changed during the treatment as follows: 4 Close the hose clamp. 4 Pull the hose and puncture needle out of the empty coolant container. -
Page 48: Reprocessing Steps In Accordance With Iso 17664
Instructions for use EXPERTsurg LUX – 1.008.3500 6 Reprocessing steps in accordance with ISO 17664 | 6.1 Manual processing 6 Reprocessing steps in accordance with ISO 17664 NOTE The reprocessing steps for surgical motors with motor cable and for the straight and contra-angle handpieces are described in the corresponding In- structions for use. - Page 49 Instructions for use EXPERTsurg LUX – 1.008.3500 6 Reprocessing steps in accordance with ISO 17664 | 6.1 Manual processing 4 Pull the button bar ① including the pump button, program button, and mo- tor direction button slightly upwards and take it off the foot control.
-
Page 50: Manual Disinfection
Approved disinfectants (application range in accordance with the available man- ufacturer's instructions for use and national guidelines. Please note material safety data sheets.) KaVo recommends the following products based on the compatibility of the materials. The microbiological efficacy must be ensured by the disinfectant manufacturer. -
Page 51: Automated Processing
The drying procedure is usually part of the cleaning program of the washer dis- infector. In order to prevent impairment of the KaVo medical device, make sure that the inside and outside of the device is dry after the end of the cycle. -
Page 52: Packaging
Instructions for use EXPERTsurg LUX – 1.008.3500 6 Reprocessing steps in accordance with ISO 17664 | 6.3 Packaging 6.3 Packaging NOTE The sterile goods package must be large enough to accommodate the product without stretching the packaging. The quality and use of the sterilization... -
Page 53: Storage
Instructions for use EXPERTsurg LUX – 1.008.3500 6 Reprocessing steps in accordance with ISO 17664 | 6.5 Storage The medical device has a maximum temperature resistance up to 138 (280.4 The following parts of the unit are released for sterilization: ▪... -
Page 54: Run A Software Update
Instructions for use EXPERTsurg LUX – 1.008.3500 7 Run a software update 7 Run a software update Please proceed as follows to update the software: 4 Download the current firmware file from www.kavo.com/ae/expertsurg. 4 Copy the firmware file to an SD card (storage capacity 1 - 32 GB, FAT for- mat). -
Page 55: Accessories
Instructions for use EXPERTsurg LUX – 1.008.3500 8 Accessories 8 Accessories The following accessories are approved for the EXPERTsurg LUX: ▪ Hose set sterile S600 (10 pcs.) (1.009.8757) ▪ Hose set sterilizable S600 (1.011.0633) ▪ Handpiece holder (1.009.3411) ▪ Motor INTRA LUX S600 LED (1.008.8000) ▪... -
Page 56: Safety Check
The EXPERTsurg LUX should be subjected to a service check including a safety check every 2 years. The safety check may only be done by a professional trained by KaVo or in a shop trained by KaVo. Perform the safety check as de- scribed in the KaVo technician's instructions. -
Page 57: 10Troubleshooting
KaVo spare parts comply with the specification. NOTE If a repair is done with NON-KaVo original spare parts, this may constitute a product modification that leads to the loss of CE conformity. In the event of damage, the responsibility is with the service company or the operator. - Page 58 Instructions for use EXPERTsurg LUX – 1.008.3500 10 Troubleshooting Malfunction Cause Remedy 4 Clean the spray nozzles with the nozzle Insufficient coolant flow in the Spray nozzles are crusty instrument. or soiled. needle or process the part. Also refer to: Instructions for use...
- Page 59 4 Arrange a precautionary appointment at a KaVo subsidiary or with a KaVo-autho- rized dealer. Service symbol is yellow. Service period is expired. 4 Arrange an appointment at a KaVo sub- sidiary or with a KaVo-authorized dealer. Service symbol is red. Service overdue: > 4...
-
Page 60: 11Decommissioning
Instructions for use EXPERTsurg LUX – 1.008.3500 11 Decommissioning | 11.1 Disconnecting the electrical connection 11 Decommissioning WARNING Dispose of the product in the appropriate manner. Infection hazard. 4 Process the product and accessories before disposal. Also refer to: 6 Reprocessing steps in accordance with ISO 17664, Page 48 11.1 Disconnecting the electrical connection... -
Page 61: Disconnecting The Surgical Motor
Instructions for use EXPERTsurg LUX – 1.008.3500 11 Decommissioning | 11.3 Disconnecting the surgical motor 4 Close the hose clamp ①. 4 Pull the puncture needle ② out of the coolant container. 4 Open the lock ③ and remove the hose. -
Page 62: Disconnecting The Foot Control
Instructions for use EXPERTsurg LUX – 1.008.3500 11 Decommissioning | 11.4 Disconnecting the foot control 11.4 Disconnecting the foot control 4 Disconnect the plug of the foot control from the connector on the device. Make sure to grasp the plug as close to the device as possible. -
Page 63: 12Disposal
Dispose of the packaging properly in accordance with the current packaging law using disposal companies/recycling firms. Comply with the comprehensive re- turn system. KaVo has had its packaging licensed for this purpose. Please com- ply with the regional public waste-disposal system. -
Page 64: 13Information Concerning The Electromagnetic Compatibility According To Iec 60601-1-2
Instructions for use EXPERTsurg LUX – 1.008.3500 13 Information concerning the electromagnetic compatibility according to IEC 60601-1-2 | 13.1 Operating environ- ment and EMC warning notes 13 Information concerning the electromagnetic compatibility according to IEC 60601-1-2 13.1 Operating environment and EMC warning notes This product is neither life-sustaining nor coupled to the patient. - Page 65 Instructions for use EXPERTsurg LUX – 1.008.3500 13 Information concerning the electromagnetic compatibility according to IEC 60601-1-2 | 13.2 Results of the elec- tromagnetic tests Requirement Class / test level DIN EN 61000-3-2 VDE 0838-2 / 03.2010 Harmonics Class A DIN EN 61000-3-3 VDE 0838-3 / 03.2014...
- Page 66 Instructions for use EXPERTsurg LUX – 1.008.3500 13 Information concerning the electromagnetic compatibility according to IEC 60601-1-2 | 13.2 Results of the elec- tromagnetic tests Requirement Class / test level Voltage interruptions, short-term interruptions and fluctuations of the supply voltage Electrical cables 0 % / 0.5 cycle...
-
Page 67: 14Terms And Conditions Of Warranty
KaVo shall not be liable for defects and their consequences that have arisen or may arise from natural wear, improper handling, cleaning, maintenance or ser-...














Need help?
Do you have a question about the EXPERTsurg LUX and is the answer not in the manual?
Questions and answers