Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for Miltenyi Biotec CliniMACS Plus
- Page 1 CliniMACS® Plus Instrument User Manual...
- Page 2 CliniMACS, MACS, and the Miltenyi Biotec logo are registered trademarks or trademarks of Miltenyi Biotec B.V. & Co. KG and/or its affiliates in various countries worldwide. All other trademarks mentioned in this document are the property of their respective...
- Page 3 CliniMACS® Plus Instrument User Manual Software version 2.4x Issued: 2022-11 210-003-188/02 Miltenyi Biotec B.V. & Co. KG Friedrich-Ebert-Straße 68 51429 Bergisch Gladbach Germany Miltenyi Biotec Technical Support +49 2204 8306-3803 technicalsupport@miltenyi.com www.miltenyibiotec.com...
- Page 5 Essential information This user manual provides information for the use of the CliniMACS Plus Instrument. For further details on the processes running on the instrument, refer to the CliniMACS Plus Applications User Manual. The operation of the CliniMACS Plus System must be performed by trained operators only.
-
Page 7: Table Of Contents
Graphical depiction Glossary of symbols 2.2.1 Safety symbols 2.2.2 Symbols used for labeling products Glossary of terms Important safety information Safety instructions for the CliniMACS Plus Instrument 3.1.1 Usage and installation 3.1.2 Electric hazards 3.1.3 Strong magnetic field 3.1.4 Biological hazards 3.1.5... - Page 8 4.10 Disposal 4.11 Description 4.12 Installation 4.12.1 Connect and switch on the CliniMACS Plus Instrument 4.12.2 Language selection and service menu 4.13 General set-up menus The CliniMACS Plus System CliniMACS Plus Instrument CliniMACS Reagents and Biotin Conjugates CliniMACS Plus Tubing Sets...
-
Page 9: Introduction
INTRODUCTION Introduction General information The CliniMACS Plus System offers a set of tools making high quality standard cell separations available for therapeutic applications. LIMITED WARRANTY Should the CliniMACS Plus System be used in a manner not explicitly described in this manual, all warranties will be null and void. -
Page 10: Clinimacs Plus Instrument Information
INTRODUCTION CliniMACS Plus Instrument information Record below the serial number located on the back of the CliniMACS Plus Instrument. Refer to these numbers when calling Miltenyi Biotec Technical Support to obtain information or request service on the instrument. Model no:... -
Page 11: Glossary
GLOSSARy Glossary Graphical depiction The following chart depicts the panels used in this user manual to inform the user about potential risks if the outlined warnings and precautions are not followed. The hazard level classifies the hazard, as described below. The level, type, and source of the hazard, as well as potential consequences, prohibitions, and measures are indicated as follows. -
Page 12: Glossary Of Symbols
GLOSSARy Glossary of symbols 2.2.1 Safety symbols General warning sign Warning: Electricity Warning: Magnetic field Warning: Biological hazard Warning: Crushing of hands 2.2.2 Symbols used for labeling products European conformity approval with ID number 0123 (ID number of Notified Body: “TÜV SÜD Product Service GmbH, Munich”) Medical Device Eurasian Conformity mark... - Page 13 GLOSSARy Date of manufacture Non-ionizing radiation Separate collection for waste of electrical and electronic equipment Fuse Keep dry Fragile, handle with care This way up Power OFF Power ON Do not re-use. Do not use if package is damaged The packaging is PVC free Use-by date Temperature limit Non-pyrogenic fluid path...
- Page 14 GLOSSARy Part number Contents of the packaging Catalogue number Serial number Unique Device Identification Sterilized using ethylene oxide Sterilized using steam or dry heat Single sterile barrier system Single sterile barrier system with protective packaging outside Phone E-Mail Website...
-
Page 15: Glossary Of Terms
Compartment of the CliniMACS Plus Instrument in which the Negative Fraction Bag and Buffer Waste Bag are placed Bag hanger Support on the CliniMACS Plus Instrument to mount the Cell Preparation Bag, Non-Target Cell Bag, Priming Waste Bag, Reapplication Bag, and buffer bag... - Page 16 Tubing Set LS, serves as filter to trap cells having non-specific interactions with the column matrix Pre-column holder Support mounted on the CliniMACS Plus Instrument that holds the pre-column in place Pump safety switch Sensor that prevents pump operation when the pump door is...
-
Page 17: Important Safety Information
Before putting the system into operation carefully read and understand the safety information, warnings, precautions, and instructions for proper operation of the CliniMACS Plus System provided in this user manual (including without limitation the safety information in this chapter) and in any safety-related recommendations issued by Miltenyi Biotec. - Page 18 Safety and performance of the instrument may be compromised. Never use the instrument with consumables, accessories, and/or cables other than those approved by Miltenyi Biotec to ensure safe and proper operation of the instrument. Note: The use of consumables, accessories, and/or cables not expressly approved by Miltenyi Biotec could void the warranty and/or invalidate the authority to operate this instrument under applicable regulations.
-
Page 19: Safety Instructions For The Clinimacs Plus Instrument
The CliniMACS Plus Instrument may be used repeatedly. It is not intended for disposal after single use. Contact the local authority governing electrical power supply, building construction, maintenance, or safety for more information regarding the installation of the equipment. -
Page 20: Electric Hazards
3.1.2 Electric hazards The CliniMACS Plus Instrument is a Protection class I instrument and may only be plugged into an outlet with a grounded connection. Risk of electric shock, electrical short, and spread of fire. Electric shock may lead to severe personal injury or death. - Page 21 The instrument should only be operated from a power source indicated on the product’s electrical ratings label. For questions about the type of power source to use, contact the authorized local Miltenyi Biotec service provider or local power company. Do not use extension cords or power strips. Do not overload an electrical outlet.
-
Page 22: Strong Magnetic Field
If hazardous material has been used or spilled, take care to thoroughly decontaminate the instrument. After running the sample and prior to decontamination, the CliniMACS Plus Instrument should be treated as a biohazard (see section“Cleaning and disinfection” on page 28). Waste disposal must be in accordance with local regulations. -
Page 23: Servicing And Transport
Risk of internal damage. Internal damage can occur if the instrument is subjected to excessive vibration or is dropped. The instrument should be transported with care in packaging specified by Miltenyi Biotec. Do not lift the instrument by the touchscreen, the peristaltic pump unit, or the magnet unit. - Page 24 IMPORTANT SAFETy INFORMATION...
-
Page 25: The Clinimacs Plus Instrument
CliniMACS Plus Cell Separation System to enable the in vitro separation of specific human cells for clinical applications. For the operation of the CliniMACS Plus System, only the stated components as CE- marked medical device and accessories defined in the CliniMACS Plus Applications User Manual for the respective applications must be used with the CliniMACS Plus Instrument. -
Page 26: Technical Data
Any serious incident that has occurred in relation to this product should be reported to Miltenyi Biotec B.V. & Co. KG - using the contact information provided - and the competent authority of the member state in which the user of this product is established. -
Page 27: Power Connection
THE CLINIMACS PLUS INSTRUMENT Protection class The instrument is a protection class I instrument (acc. to DIN 61140) and may only be plugged into an outlet with a grounded conductor. The protection category according to DIN EN 60529 is IPX 0. -
Page 28: Unpacking
Unpacking and installation of the CliniMACS Plus Instrument must only be performed by an authorized Miltenyi Biotec representative. Visually inspect and note any significant damage to the package. -
Page 29: Transport
Read the chapter 3 “Important safety information” on page 15 to avoid the risk of overheating. Risk of serious harm and/or serious damage to the instrument. The CliniMACS Plus Instrument could fall and cause serious harm and/or serious damage to the instrument if placed on an unstable surface. -
Page 30: Cleaning And Disinfection
H Clean the labels of CliniMACS Plus Instrument with a mild detergent only. IMPORTANT The surface of the CliniMACS Plus Instrument should be cleaned at regular intervals and after each application. Cleaning after unpacking and installation is also recommended. Maintenance Risk of hazards to users, unpredictable results, instrument malfunction or damage, premature wear and reduced life time of the instrument. -
Page 31: Disposal
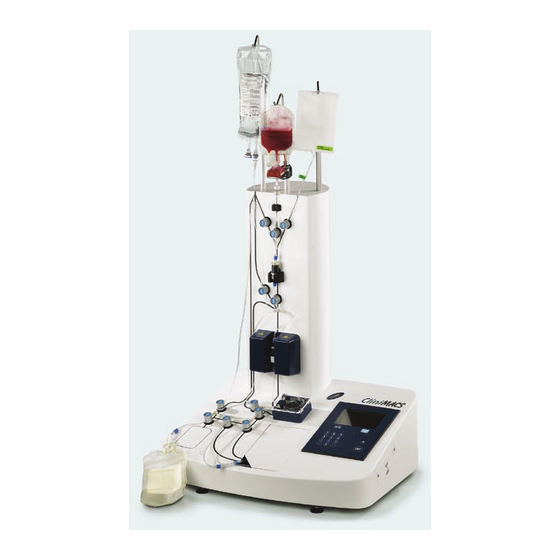
Eleven pinch valves ensure controlled flow of buffer and cell suspension throughout the procedure. The CliniMACS Plus Instrument and CliniMACS Tubing Sets allow the operator to perform cell separations in a closed and sterile system. The CliniMACS Plus Software offers the choice between various separation... - Page 32 Bag hanger Non-Target Cell Bag for Cell Preparation Bag Bag hanger clamp Liquid sensor Pinch valve Separation coliumn holder Magnetic separation unit Main ON/OFF Pump door switch Peristaltic pump Display with touchscreen compartment Figure 4.4: The CliniMACS Plus Instrument (model CS3)
-
Page 33: Installation
Risk of damage to the instrument. Risk of damage to the instrument if the instrument is installed by unauthorized persons. Installation of the CliniMACS Plus Instrument must only be performed by an authorized Miltenyi Biotec representative. Read the chapter 3 “Important safety information” on page 15 before installation and use of the instrument. -
Page 34: Language Selection And Service Menu
THE CLINIMACS PLUS INSTRUMENT 4.12.2 Language selection and service menu The CliniMACS Plus Instrument provides a menu to set-up the language and a service menu (see section 4.13). To enter these menus, wait until Screen 4.1 appears in the display window and do not press ENT as shown on the window display. - Page 35 Access to programs This program allows for the activation of additional separation programs by Miltenyi Biotec authorized personnel. Contact the Miltenyi Biotec Technical Support for further information and instructions to continue. If this program has been entered by mistake, press STOP to leave the program.
- Page 36 THE CLINIMACS PLUS INSTRUMENT Check instrument In case of a suspected malfunction of the instrument contact the Miltenyi Biotec Technical Support. If an instrument check is indicated the specialist will assist the operator in performing the instrument check sequence. IMPORTANT Important data collected by the instrument software during a CliniMACS Plus Separation are saved within the process code.
-
Page 37: The Clinimacs Plus System
CliniMACS Plus Tubing Sets The CliniMACS Tubing Sets are sterile, single-use disposables designed to be used in combination with the CliniMACS Plus Instrument for the in vitro enrichment or depletion of human cells from heterogeneous haematologic cell populations. CliniMACS PBS/EDTA Buffer The CliniMACS PBS/EDTA Buffer is intended as wash and transport fluid to enable the separation of human cells. - Page 38 THE CLINIMACS PLUS SySTEM...
-
Page 39: Troubleshooting
TROUBLESHOOTING Troubleshooting Miltenyi Biotec Technical Support In any case of instrument malfunction or process failure, contact the Miltenyi Biotec Technical Support team: +49 2204 8306-3803 technicalsupport@miltenyi.com Visit www.miltenyibiotec.com for local Miltenyi Biotec Technical Support contact information. Error messages There are a number of possible instrument or software malfunctions. - Page 40 TROUBLESHOOTING...
-
Page 41: Legal Notes
Miltenyi Biotec product, or unless otherwise agreed in writing by a duly authorized Miltenyi Biotec representative, Miltenyi Biotec’s warranty for products purchased directly from Miltenyi Biotec shall be subject to the terms and conditions of sale under which it was provided to you by the respective Miltenyi Biotec sales organization. -
Page 42: Trademarks
Trademarks CliniMACS, MACS, and the Miltenyi Biotec logo are registered trademarks or trademarks of Miltenyi Biotec B.V. & Co. KG and/or its affiliates in various countries worldwide. All other trademarks mentioned in this document are the property of their respective owners and are used for identification purposes only. -
Page 43: Appendix
Guidance and manufacturer’s declaration – Electromagnetic emissions The CliniMACS Plus Instrument is intended for the use in the professional facility healthcare environment. The instrument is not intended to be used near active HF surgical equipment. The customer or user of the instrument should assure that it is used in such an environment. - Page 44 APPENDIX Guidance and manufacturer’s declaration – Electromagnetic immunity The CliniMACS Plus Instrument is intended for use in the electromagnetic environment specified below. The customer or the user of the instrument should assure that it is used in such an environment.
- Page 45 APPENDIX Guidance and manufacturer’s declaration – Electromagnetic immunity The CliniMACS Plus Instrument is intended for use in the electromagnetic environment specified below. The customer or the user of the instrument should assure that it is used in such an environment.
- Page 46 Guidance and manufacturer’s declaration – Electromagnetic immunity The CliniMACS Plus Instrument is intended for the use in the professional facility healthcare environment. The instrument is not intended to be used near active HF surgical equipment. The customer or user of the instrument should assure that it is used in such an environment.
- Page 47 Recommended separation distances between portable and mobile RF communications equipment and the CliniMACS Plus Instrument The CliniMACS Plus Instrument is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the instrument can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters), including RFID readers, and the instrument –...
- Page 48 Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the CliniMACS Plus Instrument, including cables specified by the manufacturer. Otherwise, degradation of the performance of this...
- Page 49 Miltenyi Biotec provides products and services worldwide. Visit www.miltenyibiotec.com/local to find your nearest Miltenyi Biotec contact. CliniMACS, MACS, and the Miltenyi Biotec logo are registered trademarks or trademarks of Miltenyi Biotec B.V. & Co. KG and/or its affiliates in various...











Need help?
Do you have a question about the CliniMACS Plus and is the answer not in the manual?
Questions and answers