Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Miltenyi Biotec CliniMACS Prodigy
- Page 1 CliniMACS Prodigy® User Manual...
- Page 2 CentriCult, CliniMACS, CliniMACS Prodigy, MACS, the Miltenyi Biotec logo, PepTivator, and TexMACS are registered trademarks or trademarks of Miltenyi Biotec B.V. & Co. KG and/or its affiliates in various countries worldwide. All other trademarks mentioned in this document are the property of their respective owners and are used for identification purposes only.
- Page 3 CliniMACS Prodigy® User Manual Issued: 2021-03 38001/05 Miltenyi Biotec B.V. & Co. KG Friedrich-Ebert-Straße 68 51429 Bergisch Gladbach Germany Miltenyi Biotec Technical Support: +49 2204 8306-3803 technicalsupport@miltenyi.com www.miltenyibiotec.com...
- Page 5 Essential information This user manual provides instructions, warnings, precautions, and other important information for the use of the CliniMACS Prodigy® as well as warnings and precautions concerning the handling of biohazardous materials and cellular starting product. For details on processes run on the instrument, refer to the CliniMACS Prodigy User Manual for the respective application.
-
Page 7: Table Of Contents
Glossary of symbols 2.2.1 Safety symbols 2.2.2 Symbols used for labeling of products 2.2.3 Glossary of terms Important safety information Safety instructions for the CliniMACS Prodigy® 3.1.1 Usage and installation 3.1.2 Electric hazards 3.1.3 Strong magnetic field 3.1.4 Optical radiation hazards 3.1.5... - Page 8 Table of contents (continued) The CliniMACS Prodigy® Regulatory information 4.1.1 The CliniMACS Prodigy® within the CliniMACS Prodigy Cell Separation System 4.1.2 The CliniMACS Prodigy® within the CliniMACS Prodigy Cell & Gene Therapy Manufacturing System Technical data Components of the CliniMACS Prodigy®...
- Page 9 Table of contents (continued) Unpacking and installation 4.5.1 Scope of supply 4.5.2 Transport 4.5.3 Positioning Cleaning and disinfection Maintenance Disposal The CliniMACS Prodigy® Software Touchscreen 5.1.1 Use of touchscreen 5.1.2 General setup menu Login, logout, and emergency stop 5.2.1 Login 5.2.2 Logout 5.2.3...
- Page 10 Table of contents (continued) The CliniMACS Prodigy® System The CliniMACS Prodigy® System components Additional materials and equipment Limitation Warnings and precautions regarding the process Warnings and precautions regarding the handling of biohazardous material Warnings and precautions regarding the cellular starting product...
-
Page 11: Introduction
Introduction 1.1 General information The CliniMACS Prodigy System offers a set of tools that enables high-quality and standardized cell processing for many different applications. The system is based on the magnetic cell separation technology (MACS® Technology) developed by Miltenyi Biotec. -
Page 12: Service Information
Reference no. (REF): Serial no. (SN): The software version is shown during the start-up phase of the instrument. 1.2.2 Technical support For information or support, contact Miltenyi Biotec Technical Support: Miltenyi Biotec B.V. & Co. KG Friedrich-Ebert-Straße 68 51429 Bergisch Gladbach... -
Page 13: Glossary
Glossary 2.1 Graphical depiction The following chart depicts the panels used in this user manual to inform the user about potential risks if the outlined warnings and precautions are not followed. The hazard level classifies the hazard, as described below. The level, type, and source of the hazard, as well as potential consequences, prohibitions, and measures are indicated as follows. -
Page 14: Glossary Of Symbols
2.2 Glossary of symbols 2.2.1 Safety symbols General warning sign Warning: Electricity Warning: Magnetic field Warning: Optical radiation Warning: Biological hazard Warning: High sound pressure level Warning: Crushing of hands Warning: Hot surface... -
Page 15: Symbols Used For Labeling Of Products
2.2.2 Symbols used for labeling of products Medical Device European conformity approval with ID number 0123 (ID number of Notified Body: “TÜV SÜD Product Service GmbH, Munich”). Eurasian Conformity mark NRTL certification mark: Product meets consensus-based standards of safety, required by the Occupational Safety/ Health Administration (OSHA), determined by the Nationally Recognized Testing Laboratories (NRTL) TÜV SÜD Consult instructions for use... - Page 16 Keep dry Fragile, handle with care This way up Power OFF Power ON Installation date Do not re-use. Do not use if package is damaged The packaging is PVC free Use-by date Temperature limit Non-pyrogenic fluid path Batch code Part number Contents of the packaging...
- Page 17 Catalogue number Serial number Unique Device Identification Sterilized using aseptic processing techniques Sterilized using steam or dry heat Sterilized using irradiation Non-sterile Single sterile barrier system Single sterile barrier system with protective packaging outside Phone E-Mail Website...
-
Page 18: Glossary Of Terms
Compartment of the CliniMACS Prodigy for the storage of bags during instrument operation Bag hanger Component of the CliniMACS Prodigy on which elements of the CliniMACS Prodigy Tubing Sets, such as buffer bags, application bags, or vials are mounted Bar code Machine-readable representation of data, e.g., part... - Page 19 Part of the CentriCult Unit to control the centrifugation speed Chamber Compartment for fractionation, washing, and labeling by centrifugation and for cultivation of cells, part of the CliniMACS Prodigy Tubing Sets Chamber drive unit Unit which drives the chamber within the CentriCult Unit Chamber lock adapter...
- Page 20 Cleaning solution bag Bag containing the cleaning solution. The cleaning solution bag is not part of the CliniMACS Prodigy Tubing Sets. CliniMACS PBS/EDTA Buffer used for cell preparation and cell separation Buffer with the CliniMACS System: PBS (phosphate- buffered saline), supplemented with 1 mM EDTA, pH 7.2.
- Page 21 Three gas inlet connectors at the rear side of the instrument for the connection of external gas supplies Gas mix unit The gas mix unit inside the CliniMACS Prodigy is used to prepare a mixture of up to three different gases for cell culture purpose. Only CO , compressed...
- Page 22 The infusion solution is not part of the CliniMACS Prodigy System. Infusion solution bag Bag containing the infusion solution. The infusion solution bag is not part of the CliniMACS Prodigy Tubing Sets.
- Page 23 CentriCult Unit Leukapheresis Apheresis collecting leukocytes Liquid sensor The CliniMACS Prodigy is equipped with four liquid sensors that monitor the flow of liquid in the tubing set based on an ultrasonic principle. It allows detection of air bubbles.
- Page 24 Instrument part used to control the flow rate of fluid within the tubing set Pinch valve Part of the CliniMACS Prodigy: 24 magnetic valves are used to fix the CliniMACS Prodigy Tubing Set onto the instrument and to ensure controlled flow pathways within the tubing set throughout the procedure...
- Page 25 Medium for cultivation and processing of cells within the tubing set Pump safety switch Sensor that prevents pump operation when the pump door is open, part of the CliniMACS Prodigy QC Bag Bag containing automatically collected in-process QC-samples, part of specific CliniMACS Prodigy...
- Page 26 Transfer bag Bag with a tubing and a spike at the end. The transfer bag is not part of the CliniMACS Prodigy Tubing Sets. TubeSealer port Connector for the MACS TubeSealer...
- Page 27 Vial adapter Adapter on the CliniMACS Prodigy Tubing Sets to connect the vial (e.g. CliniMACS Reagent vial) Vial holder Part of the bag hanger assembly to hold a vial in...
-
Page 29: Important Safety Information
Important safety information The operation of the CliniMACS Prodigy System must be performed by trained operators only. Before putting the system into operation, care fully read and understand the safety information, warnings, precautions, and instructions for proper operation of the CliniMACS Prodigy provided... - Page 30 Safety and performance of the instrument may be compromised. Never use the instrument with consumables, accessories, transducers, and/or cables other than those approved by Miltenyi Biotec to ensure safe and proper operation of the instrument. Note: The use of consumables, accessories, transducers, and/or cables not •...
- Page 31 Failure to comply with the safety information, warnings, precautions, and instructions in the user manuals (and in other safety related publications issued by Miltenyi Biotec for use with the instrument) could lead to improper or incorrect use, handling or...
-
Page 32: Safety Instructions For The Clinimacs Prodigy
Miltenyi Biotec Service Provider. The CliniMACS Prodigy may be used repeatedly. It is not intended for disposal after single use. Contact the local authority governing electrical power supply, building construction, maintenance, or safety for more information regarding the installation of the equipment. -
Page 33: Electric Hazards
Contact Miltenyi Technical Support. An electrical short and overheating may lead to the spread of fire. The CliniMACS Prodigy is a Protection Class I device and may only be plugged into an outlet with a grounded connection. Electronic equipment may emit sparks that could ignite combustible vapors or dusts leading to explosion or spread of fire. - Page 34 The instrument should only be operated from a power source indicated on the product’s electrical ratings label. For questions about the type of power source to use, contact the authorized local Miltenyi Biotec Service Provider. Do not use extension cords or power strips. Do not overload an electrical outlet.
-
Page 35: Strong Magnetic Field
3.1.4 Optical radiation hazards The CliniMACS Prodigy is equipped with a light emitting diode (LED) for the signal lamp at the left bag hanger (position A). The optical radiation emitted from the LED may be harmful to the eyes at close viewing distances. -
Page 36: Chemical And Biological Hazards
Imbalance during centrifugation can lead to substantial vibrations. Shut down the instrument when substantially strong vibrations occur. Contact Miltenyi Biotec Technical Support for assistance. Keep away from all moving parts. Do not lean on the CliniMACS Prodigy or the CentriCult Unit. -
Page 37: Gas Hazards
Unless otherwise specifically noted in this user manual, do not service the CliniMACS Prodigy. Service and repair may only be performed by authorized local Miltenyi Biotec Service Provider. - Page 38 Risk of internal damage. Internal damage can occur if the instrument is subjected to excessive vibration or is dropped. The instrument should be transported with care in packaging specified by Miltenyi Biotec. Do not lift the instrument by the touchscreen, the CentriCult Unit, peristaltic pump unit, or the magnet unit.
-
Page 39: Position Of Safety Symbols
3.2 Position of safety symbols Notice the positions of the safety symbols (see Figure 3.1 and Figure 3.2) on the CliniMACS Prodigy and keep them in an easily readable state. The safety labels must be kept clean and legible. Magnetic field... -
Page 41: The Clinimacs Prodigy
The CliniMACS Prodigy Cell Separation System is intended to separate human cells. CliniMACS Prodigy is a Medical Device in Europe if used as part of the CliniMACS Prodigy Cell Separation System. The CliniMACS Prodigy Cell & Gene Therapy Manufacturing System is intended for genetic and/or other substantial manipulation (like proliferation, differentiation) steps of human cells. -
Page 42: The Clinimacs Prodigy® Within The Clinimacs Prodigy Cell Separation System
The CliniMACS Prodigy is intended to operate the components of the CliniMACS Prodigy Cell Separation System to enable the in vitro separation of specific human cells for clinical applications. -
Page 43: The Clinimacs Prodigy® Within The Clinimacs Prodigy Cell & Gene Therapy Manufacturing System
Any serious incident that has occurred in relation to this product should be reported to Miltenyi Biotec B.V. & Co. KG – using the contact information provided – and the competent authority of the member state in which the user of this product is established. -
Page 44: Technical Data
Safety and performance of the CliniMACS Prodigy may be compromised. Safety and performance of the instrument may be compromised if the CliniMACS Prodigy is used outside its specifications. Do not use the instrument outside its specifications. The technical data of the instrument are listed in Table 4.1. - Page 45 • Consult the dealer or an experienced radio/TV technician for help. Changes or modifications of the instrument, unless expressly approved by Miltenyi Biotec, may void the authority to operate the instrument pursuant to FCC 47 CFR.
-
Page 46: Components Of The Clinimacs Prodigy
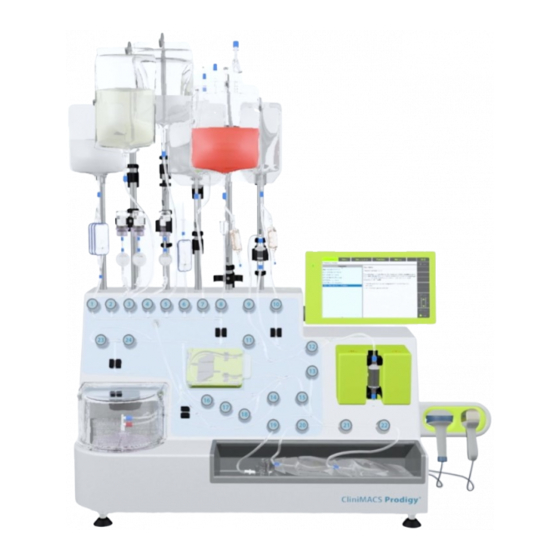
4.3 Components of the CliniMACS Prodigy® The following section describes the components of the CliniMACS Prodigy®. Table 4.2 gives an overview of components included per instrument serial number. Repairs or hardware updates made by the authorized local Miltenyi Biotec Service Provider may lead to a modified state of construction and retrofitting of the instrument. - Page 47 The front view of the instrument is shown in Figure 4.1. Liquid Monitor with touchscreen hanger outlet sensor (LS) Magnet unit USB ports Bar code reader (accessory) Peristaltic Pinch pump valve MACS CentriCult Supplementary Bag TubeSealer Unit compartment compartment (accessory) Figure 4.1: Front view of the instrument (exemplary SN 491 or higher)
- Page 48 The rear view of the instrument is shown in Figure 4.2. Main Gas inlets and safety valve power Instrument Connector Protective label label panel earthing Figure 4.2: Rear view of the instrument (exemplary SN 491 or higher)
-
Page 49: Housing And Bag Hangers
(e.g. waste bags). The touchscreen is also connected to the housing. The CliniMACS Prodigy is equipped with six bag hangers labeled A to F at the top of the instrument (see Figure 4.3). An individual bag hanger is comprised of a rod, a spring loaded pinch clamp with a hook from which bags can hang, and additional supports, such as vial holders and pre-column holders. -
Page 50: Monitor
4.3.2 Monitor The user interface is operated via touchscreen and manages all functions of the CliniMACS Prodigy. Usage of the touchscreen is possible when wearing surgical gloves. The touchscreen guides the operator through the setup procedure and allows monitoring of the instrument during operations. The monitor unit holds an internal speaker for acoustical signaling and alarm functionality. -
Page 51: Magnet Unit
4.3.4 Magnet unit The magnet unit induces a magnetic field into the separation column in order to separate magnetically labeled cells from non-labeled cells. This unit includes the movable, permanent magnet and a holder for the separation column (see Figure 4.5). A linear motor with position sensors is used to place the magnet in the ON and OFF positions. - Page 52 Positioning of the chamber and focusing of the lens is performed via the graphical user interface. Note: The microscope camera is part of the CliniMACS Prodigy Cell & Gene Therapy Manufacturing System only. Lid of CentriCult Unit...
-
Page 53: Gas Mix Unit
4.3.6 Gas mix unit Note: The gas mix unit is part of the CliniMACS Prodigy Cell & Gene Therapy Manufacturing System only. It enables the preparation of a mixture of up to three different gases for cell culture purposes. Located at the back of the CliniMACS Prodigy are three gas inlets with 4 mm push-in connectors for CO... -
Page 54: Sensors
4.3.7 Sensors A variety of sensors, such as pressure sensors and liquid sensors, are used to control the process and verify correct user handling. The pressure sensors monitor the pressure within the tubing set to allow detection of leakages or clogging within the tubing set. -
Page 55: Connector Panel
Only accessories, transducers, and cables, authorized by Miltenyi Biotec for the use in combination with the CliniMACS Prodigy, are allowed to be plugged into any of the connectors of the connector panel or any other connector socket of the instrument. - Page 56 Relay 1 Figure 4.9: Relay circuit of alarm connector showing the idle state CliniMACS Prodigy manufactured until October 2020 A connector panel with connectors for input and output connections is positioned at the rear side of the instrument (see Figure 4.10). There are three USB ports for data input and output functionalities.
-
Page 57: Accessories For The Clinimacs Prodigy
CliniMACS Materials required for an application. These numbers and any other process-related parameters may also be entered manually. The bar code reader can be connected to any USB port of the CliniMACS Prodigy. For handling reasons, it is recommended to connect the bar code reader to one of the three... -
Page 58: Clinimacs Prodigy® Supplementary Bag
The CliniMACS Prodigy® Supplementary Bag is intended to help reduce the user's contact to potentially infectious sample material which may be accidentally emitted out of the CliniMACS Prodigy Tubing Set. In case of a malfunction –especially a chamber leakage– liquid might leak into the enclosed CliniMACS Prodigy housing and will be collected inside the removable Supplementary Bag which can be disposed of easily. -
Page 59: Clinimacs® Formulation Unit
Refer to the CliniMACS Formulation Unit User Manual for further information. 4.5 Unpacking and installation Risk of damage to the CliniMACS Prodigy. Risk of damage to the instrument if the instrument is unpacked or installed by unauthorized persons. Unpacking and installation of the instrument must only be performed by an authorized local Miltenyi Biotec Service Provider. -
Page 60: Transport
4.5.2 Transport Risk of internal damage. Internal damage can occur if the instrument is subjected to excessive vibration or if it is dropped. The CliniMACS Prodigy should be transported with care in packaging specified by Miltenyi Biotec. Chemical and biological hazard. Risk of chemical or biological hazards due to contaminated surfaces. -
Page 61: Cleaning And Disinfection
4.6 Cleaning and disinfection Risk of electric shock or damage to the instrument. Risk of electric shock or damage to the instrument if the CliniMACS Prodigy is cleaned with excessive amount of cleaning agent or while switched on. Only clean the instrument when it is switched off and the power cord is unplugged. -
Page 62: Maintenance
Routine and preventative maintenance procedures should be conducted by the manufacturer’s authorized service personnel at least once a year. The CliniMACS Prodigy does not require any form of calibration. Contact the authorized local Miltenyi Biotec Service Provider for instrument service and support arrangements. -
Page 63: The Clinimacs Prodigy® Software
The CliniMACS Prodigy® Software The CliniMACS Prodigy® Software allows the operator to choose between processes and user programs and control certain instrument functionalities. The following notations are used to describe software elements: • Text in bold gray indicates elements available for selection (e.g. press... -
Page 64: General Setup Menu
5.1.2 General setup menu After switching on the power of the CliniMACS Prodigy, the software starts up and the start screen appears for a short time (approx. 5 seconds). The start screen provides information about the software version and the result of the initialization process. -
Page 65: Login
CliniMACS Prodigy. In addition, it is highly recommended to establish a so-called “break-glass solution” for emergency situations allowing the login for an individual user lacking sufficient access rights. -
Page 66: Emergency Stop
5.2.3 Emergency Stop « Emergency Stop » functionality enables an immediate pause of a running application or a tool in case of an emergency situation when no user is logged in, without violating 21 CFR Part 11 requirements to the audit trail. In such a case, the software requests a reason for the emergency stop and a subsequent login. - Page 67 Menu bar Status line List of processes Text field Tool bar Figure 5.2: Operating controls (up to software version 1.4) Menu bar The software offers several menu buttons for different functionalities (see Figure 5.2). A menu is selected by pressing the respective menu button. A green background indicates which menu button is active.
- Page 68 Application Services Figure 5.3: New Application Services button in menu bar as of software version 2.0 Lists Lists consist of similar elements, e.g., list of processes (see Figure 5.2) or user programs. Each list element can be chosen and marked by directly pressing the element or by using the «...
- Page 69 Tool bar The tool bar shows the changing functions of the touch buttons and is divided in a dynamic and static part. The static buttons of the tool bar are the « stop », navigation, « undo », « edit », and «...
- Page 70 Static buttons The functions of the static buttons (see Figure 5.4) do not change with respect to the different menus. The only exception is the « ok » button which changes into a « run » button whenever there is a process, a user program, or any other procedure to be started.
- Page 71 Dynamic buttons The functions of the buttons (see Figure 5.4) in the dynamic part will change according to the activated menu and the currently running application. • Open lid button « open lid » button enables the customer to manually open the CentriCult Unit lid.
-
Page 72: Menus
5.3.2 Menus Processes menu After start up and initialization of the CliniMACS Prodigy Software, the menu Processes is selected automatically. The central element of this menu is the list of processes that can be performed (see Figure 5.2, left light blue-colored window). - Page 73 Graphic overview • The graphic overview element (see Figure 5.6, lower right window) provides visual information about the running subprocess status of the instrument. All activities of the instrument components (peristaltic pump, pinch valves, etc.) are visualized in orange. In this menu, the tool bar offers additional functions.
- Page 74 Layer Detection Camera The camera menu shows the last image saved by the Layer Detection Camera. Red and orange bars mark the position of the layers within the image. A diagram below the image continuously displays the recorded values for the volumes of the centrifugation fractions.
- Page 75 Microscope camera Note: The microscope camera is part of the CliniMACS Prodigy Cell & Gene Therapy Manufacturing System only. It is only active in processes with cell culture procedures. The magnification of the images is up to 400-fold. As with the Layer Detection Camera, the camera menu shows the last image saved by the integrated microscope camera (see Figure 5.8).
- Page 76 Figure 5.8: Status screen when microscope camera is active...
- Page 77 User programs menu Note: The microscope camera is part of the CliniMACS Prodigy Cell & Gene Therapy Manufacturing System only. The menu User programs offers space for generic, pre-installed programs as well as for customized tailor-made programs. Customized programs can be requested from the Miltenyi Biotec Customized Application (CAP) Service.
- Page 78 Filed data menu Filed data The menu contains a list of protocols indicating all processes executed on the instrument. The list provides information regarding the relevant parameters of the individual process runs, for example, the date and duration of a process. Note: The menu Filed data is not available during a process run.
- Page 79 Settings menu In the menu Settings, the operator will find additional programs for configuration and survey of the instrument. When the menu is selected, three columns are displayed. The active window is indicated by a light blue background color. All programs in this menu are grouped in different categories (Option groups) that are listed in the left window.
-
Page 80: Application Services
5.4 Application Services With the CliniMACS Prodigy software version 2.0, new functionalities have been integrated as the “Application Services” into the software. These services comprise a user management module including authentication features and an audit trail module which are essential to support 21 CFR Part 11 compatibility. -
Page 81: User Management
5.4.1 User Management To establish the user management functionality on the CliniMACS Prodigy, it is necessary to create individual accounts for all users of a given instrument and to define their individual set of roles. In principal, there are two main user categories: operators and administrators. - Page 82 Note: The submenu LDAP Groups is not active for use in software version 2.0. In addition, the displayed account “cmp-service” in the example shown in Screen 5.2 represents a pre-installed account for Miltenyi Biotec Instrument Service staff. Such accounts cannot be deleted nor adapted.
- Page 83 « + New User » Pressing leads to a new submenu for the creation of a new account with its own ID, user details, password, and the assignment of specific Role roles. All assignable roles are listed in the first tab under “Available Roles”...
- Page 84 The password to this account has to be assigned by opening the tab Authentication. The password must be set according to the password policy Configuration Password which may be adapted in the submenu under tab Policies. Further user details, e.g. name, initials etc., can be defined by opening the third tab Local User Details.
- Page 85 « + Add New Role ». entered by pressing Note: This new window also contains rights for Miltenyi Biotec Instrument Service which are not activated for local administrators. Table 5.1 lists the different roles available in software version 2.0 and explains the tools or rights which are associated with this role.
- Page 86 Screen 5.5: Submenu “Configuration” with active “Password Policy” tab Import/Export Opening the tab allows the export of user management setting like custom roles, user setups, or password policies onto a USB device which can be used for settings on different CliniMACS Prodigy in the same facility.
- Page 87 603 events in total. By scrolling through the list all events could be checked and eventually be exported, e.g., as a PDF to a USB device inserted to the CliniMACS Prodigy using the « Create Export »...
-
Page 88: Alarm Management
Screen 5.7: Warning pop-up to delete audit trail events 5.5 Alarm management The CliniMACS Prodigy is equipped with an integrated alarm management system which is spread over several components, providing optical and acoustical alarm signals if required. An additional relay circuit may be connected to an external alarm system. - Page 89 The alarm management system of the instrument features a three-level alarm system. In case of a failure, a message is displayed on the monitor, accompanied by an audible warning signal from a speaker within the touchscreen. The signal lamp at the bag hanger A (see Figure 4.3) will then display a red blinking light. The instrument distinguishes between three alarm levels as described in Table 5.2.
-
Page 91: The Clinimacs Prodigy® System
The CliniMACS Prodigy® System 6.1 The CliniMACS Prodigy® System components The different applications run on the CliniMACS Prodigy® require the use of specific CliniMACS Prodigy System components as well as additional materials and equipment as described in the CliniMACS Prodigy User Manual for the respective application. -
Page 92: Additional Materials And Equipment
6.3 Limitation Miltenyi Biotec as the manufacturer of the CliniMACS System does not give any recommendations regarding the use of separated cells for therapeutic purposes and does not make any claims regarding a clinical benefit. -
Page 93: Warnings And Precautions Regarding The Process
All processing procedures must be performed by trained operators only. Operator training will be provided by a qualified Miltenyi Biotec representative. • For the manufacturing and use of target cells in humans the national legislation and regulations – e.g. -
Page 94: Warnings And Precautions Regarding The Handling Of Biohazardous Material
• The CliniMACS Prodigy should be considered as potential biohazard after each run and cleaned with an aqueous biocidal detergent (see section 4.6) according to standard hospital or institutional requirements. -
Page 95: Warnings And Precautions Regarding The Cellular Starting Product
6.6 Warnings and precautions regarding the cellular starting product IMPORTANT Labeling and processing of cells should begin as soon as possible after the cellular starting product has been collected. It is recommended to start all labeling and processing procedures within 24 h after the cell collection. •... -
Page 97: Troubleshooting
+49 2204 8306-3803 technicalsupport@miltenyi.com Visit www.miltenyibiotec.com for local Miltenyi Biotec Technical Support contact information. 7.2 Instrument cleaning after leakage In case leakage occurs, e.g., in the CentriCult Unit, additional cleaning measures are required. For further information contact the Miltenyi Biotec Technical Support. -
Page 99: Legal Notes
8.1 Limited warranty Except as stated in a specific warranty statement, which may accompany this Miltenyi Biotec product, or unless otherwise agreed in writing by a duly authorized Miltenyi Biotec representative, Miltenyi Biotec’s warranty for products purchased directly from Miltenyi Biotec shall be subject to the terms and conditions of sale under which it was provided to you by the respective Miltenyi Biotec sales organization. -
Page 100: Trademarks
Miltenyi Biotec‘s warranty does not cover products sold AS IS or WITH ALL FAULTS or consumables. Nothing herein should be construed as constituting an additional warranty. -
Page 101: Appendix
Miltenyi Biotec Technical Support. Guidance and manufacturer’s declaration – Electromagnetic emissions The CliniMACS Prodigy is intended for use in the electromagnetic environment specified below. The customer or the user of the instrument should assure that it is used in such an environment. - Page 102 Guidance and manufacturer’s declaration – Electromagnetic immunity The CliniMACS Prodigy is intended for use in the electromagnetic environment specified below. The customer or the user of the instrument should assure that it is used in such an environment. IEC 60601...
- Page 103 Guidance and manufacturer’s declaration – Electromagnetic immunity The CliniMACS Prodigy is intended for use in the electromagnetic environment specified below. The customer or the user of the instrument should assure that it is used in such an environment. IEC 60601...
- Page 104 Recommended separation distances between portable and mobile RF communications equipment and the CliniMACS Prodigy The CliniMACS Prodigy is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the instrument can...
- Page 105 CliniMACS Prodigy® manufactured as of 2019 EMC compliance with IEC 60601-1-2:2014 (Edition 4) has been attested for the CliniMACS Prodigy® and the approved accessories (see Table 4.3). The use of other power cables may result in increased electromagnetic emissions or decreased immunity of the CliniMACS Prodigy.
- Page 106 Guidance and manufacturer’s declaration – Electromagnetic immunity The CliniMACS Prodigy is intended for use in the electromagnetic environment specified below. The customer or the user of the instrument should assure that it is used in such an environment. Immunity test...
- Page 107 Guidance and manufacturer’s declaration – Electromagnetic immunity to RF wireless communication equipment Test Immunity Band Maximum Distance Compliance Frequency Service Modulation Test Level (MHz) Power (W) Level (V/m) (MHz) (V/m) TETRA 400 Pulse – modulation 18 Hz GMR S460, FRS – ±5 kHz deviation 1 kHz sine...
- Page 108 CentriCult, CliniMACS, CliniMACS Prodigy, MACS, the Miltenyi Biotec logo, PepTivator, and TexMACS are registered trademarks or trademarks of Miltenyi Biotec B.V. & Co. KG and/or its affiliates in various countries worldwide. Copyright © 2021 Miltenyi Biotec and/or its affiliates. All rights reserved.











Need help?
Do you have a question about the CliniMACS Prodigy and is the answer not in the manual?
Questions and answers