Summary of Contents for Verathon BFlex 2
- Page 1 BFlex Single‑Use Bronchoscopes for GlideScope Core ® Operations & Maintenance Manual...
- Page 2 0900‑5209 REV‑00...
- Page 3 BFlex 2 Single‑Use Bronchoscopes for GlideScope Core Operations & Maintenance Manual Effective: March 9, 2023 Caution: Federal (United States) law restricts this device to sale by or on the order of a physician. 0900‑5209 REV‑00...
- Page 4 Fax: +1 604 439 3039 verathon.com Copyright © 2023 by Verathon Inc. All rights reserved. No part of this manual may be copied or transmitted by any method without the express written consent of Verathon Inc. GlideScope, BFlex, Verathon, and all associated symbols are trademarks of Verathon Inc. All other brand and product names are trademarks or registered trademarks of their respective owners.
-
Page 5: Table Of Contents
Table of Contents IMPORTANT INFORMATION .............................1 Product Description ..............................1 Statement of Intended Use ............................1 Intended Patient Population ............................1 Intended Use Environment and User Population ......................1 Contraindications ................................2 Essential Performance ...............................2 Environments of Intended Use ..........................2 Statement of Prescription ............................2 Notice to All Users ..............................2 Warnings &... - Page 6 REPROCESSING ...............................22 MAINTENANCE & SAFETY ............................23 Periodic Inspections ..............................23 Device Repair ................................23 Device Disposal ................................23 WARRANTY ................................24 PRODUCT SPECIFICATIONS ..........................26 Specifications, Standards, and Approvals .......................26 Component Specifications ............................27 Electromagnetic Compatibility ..........................32 GLOSSARY .................................35 0900‑5209 REV‑00...
-
Page 7: Important Information
Intended Patient Population The BFlex 2 Single‑Use system is for use in a hospital environment. All four BFlex 2 bronchoscopes are single‑use devices designed for use in adults. BFlex 2 Ultraslim 2.8 is also designed for pediatric use and has been tested at 6 months to 6 years. -
Page 8: Contraindications
Contraindications The BFlex 2 Ultraslim 2.8 Single‑Use Bronchoscope does not have a working channel and therefore cannot be used for therapeutic purposes. Essential Performance The essential performance of the BFlex 2 Single‑Use bronchoscope is visualization of the airway and tracheobronchial tree as well as certain procedures such as suction and use of endoscopic accessories sized to work with the dimensions of the bronchoscope. - Page 9 Do not use the power adapter in the presence of flammable anesthetics. WARNING Verathon has conducted no analysis to establish the compatibility of the system with environments where magnetic resonance imaging (MRI) equipment is installed. Because of this, the owner of the system must exclude it from any magnetic resonance (MR) environment.
- Page 10 WARNING Two areas of the bronchoscope tip that contact the patient can exceed 41°C (106°F) as part of normal operation: The first area is the light‑emitting area surrounding the camera in the tip. When used as indicated, continuous contact with this area is unlikely because, if tissue were to contact this area, a usable view would be lost.
- Page 11 To maintain electrical safety, use only the provided power supply. Connect the power cord and power adapter to a properly grounded plug, and ensure that the disconnect is easily accessible. Use only the accessories and peripherals recommended by Verathon. Operations & Maintenance Manual: Important Information...
- Page 12 Portable radio frequency communications equipment (including peripherals such as antenna cables and external antennas) may not be used within 30 cm (12 inches) of any part of the BFlex 2 Single‑Use Bronchoscope system, including cables that Verathon specifies or provides for use with the system. If this distance is not maintained, performance of the system may be degraded and image display may be compromised.
- Page 13 Verathon (or its authorized representative), the Competent Authority of the Member State where the incident occurred, or both. CAUTION The BFlex 2 3.8 Single‑Use Bronchoscope should not be used with 35Fr Shiley Endobronchial Tubes. Damage or tear to the BFlex 2 tip sheath may occur. CAUTION Do not use a BFlex holder as a handle when moving the Workstation.
- Page 14 Cautions: Reprocessing CAUTION QuickConnect Cable Only: For information on the handling and disposing of recommended reprocessing solutions, please refer to the solution manufacturer’s instructions. CAUTION Risk of permanent equipment damage. This product is sensitive to heat, which causes damage to the electronics.
-
Page 15: Introduction
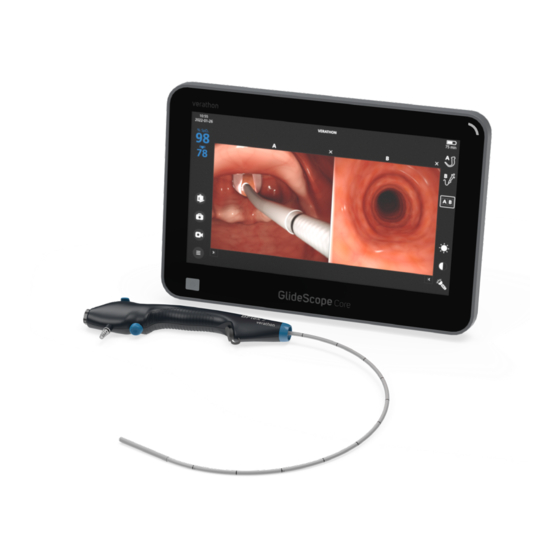
Introduction This manual discusses the following components of the BFlex 2 Single‑Use Bronchoscope system: • BFlex 2 bronchoscope (single‑use) • GlideScope Core QuickConnect Cable (reusable) Note: This manual covers the single‑use bronchoscope and the reusable cables. For information about using a video monitor, refer to that monitor's Operations & Maintenance Manual. -
Page 16: Parts & Accessories
Parts & Accessories Table 1. System Components BRONCHOSCOPES BFlex 2 BFlex 2 Ultraslim 2.8 Single‑Use bronchoscope BFlex 2 Slim 3.8 Single‑Use bronchoscope BFlex 2 Regular 5.0 Single‑Use bronchoscope BFlex 2 Large 5.8 Single‑Use bronchoscope Accessories GlideScope Core QuickConnect Cable Introducer... -
Page 17: Bronchoscope Components
Bronchoscope Components The BFlex 2 bronchoscope is a single‑use device that can be inserted either directly or through an endotracheal (ET) tube. The main components of the bronchoscope are shown in the following figure. Figure 2. Bronchoscope Components Table 2. -
Page 18: Setting Up
2. Inspect the components for damage. 3. If any of the components are missing or damaged, notify the carrier and Verathon Customer Care or your local representative. For contact information, visit verathon.com/service‑and‑support. Attach the Video Cable to the Monitor This procedure provides basic instruction on connecting video cables to a monitor. -
Page 19: Attach The Bronchoscope To The Video Cable
3. Detach the protective cover from the cable connector on the bronchoscope. Discard the cover after you remove it. 4. If you are using a BFlex holder, place the BFlex 2 with the protective sleeve in the holder. Otherwise, carefully slide the protective sleeve off the insertion tube of the bronchoscope. -
Page 20: Perform A Functional Check
Perform a Functional Check Before you use the device for the first time, perform the following functional check to ensure that the system is working properly. Please contact your Verathon Customer Care representative if your system does not function as described below. -
Page 21: Using The Device
Warnings & Cautions section before performing the following tasks. BFlex 2 Single‑Use bronchoscopes are equipped with an anti‑fog feature that reduces camera fogging during use. To optimize the feature fully, you must allow the bronchoscope to warm up for 30–120 seconds prior to use, depending on the ambient temperature and humidity of the clinical environment. -
Page 22: Procedure 1. Prepare The Glidescope System
If the bronchoscope is stored in cold conditions, additional warming time may be required for optimal performance of the anti‑fog feature. * Does not apply to BFlex 2 Ultraslim 2.8. 0900‑5209 REV‑00... -
Page 23: Procedure 2. Position The Handle And Controls
Note: To ensure full suction strength, remove any object such as a syringe or an endoscopic accessory from the working channel while applying suction. * Does not apply to BFlex 2 Ultraslim 2.8. Operations & Maintenance Manual: Using the Device... -
Page 24: Procedure 3. Insert Through A Tube Or Catheter (Optional)
Note: There is no guarantee that instruments selected solely using these instrument dimensions will be compatible in combination. Importantly, The BFlex 2 Slim 3.8 Single‑Use Bronchoscope should not be used with 35Fr Shiley Endobronchial Tubes. Damage or tear to BFlex 2 tip sheath may occur. -
Page 25: Procedure 4. Insert And Flex The Bronchoscope
The bronchoscope can be inserted using any standard oral or nasal insertion technique, with or without the use of a separate ET tube. During use, its distal tip can flex through the ranges shown in the following table. Table 4. BFlex 2 Bronchoscope—Distal Tip Articulation RANGE OF MOVEMENT SIZE OF DISTAL TIP BFlex 2 Ultraslim 2.8... -
Page 26: Procedure 5. Introduce Liquids Or Accessories (Optional)
Warnings & Cautions section before performing the following task. IMPORTANT The BFlex 2 Ultraslim 2.8 does not have suction capability. In addition to supplying suction, the working channel on the bronchoscope also provides a delivery channel for the following items: •... -
Page 27: Procedure 6. Remove The Bronchoscope
If you intend to use the bronchoscope with the same patient more than once, prepare a sterile resting area for it. If you are using a BFlex 2 with a BFlex holder, the holder can be used as an intra‑operative storage location. -
Page 28: Reprocessing
Some of the components in this manual may require cleaning, low‑level disinfection, high‑level disinfection, or sterilization between uses or under specific circumstances. For information about the cleaning, disinfection, and sterilization requirements for these components, refer to the GlideScope and GlideRite Products Reprocessing Manual, which is available at verathon.com/service‑and‑support/glidescope‑reprocessing‑products. 0900‑5209 REV‑00... -
Page 29: Maintenance & Safety
Device Repair The cables are not user‑serviceable. Verathon does not make available any type of circuit diagrams, component parts lists, descriptions, or other information that would be required for repairing the device and related accessories. All service must be performed by a qualified technician. -
Page 30: Warranty
Verathon warrants the system against defects in material and workmanship. The limited warranty applies for one (1) year from the date of shipment from Verathon and applies only to the original purchaser of the system. The terms of this warranty are subject to the Terms and Conditions of Sale or any other contractual document between the parties. - Page 31 The information, descriptions, recommendations, and safety notations in this manual are based upon Verathon experience and judgment. The contents of this manual should not be considered to be all‑inclusive or to cover all contingencies.
-
Page 32: Product Specifications
Product Specifications Specifications, Standards, and Approvals Table 6. Specifications GENERAL SPECIFICATIONS Single‑use bronchoscope IPX0 Ingress protection against water: QuickConnect Cable (all) IPX7 Refer to the “use by” date indicated by Expected product life: Single‑use bronchoscope (all) symbol on the package label. OPERATING, SHIPPING, &... -
Page 33: Component Specifications
Component Specifications Table 7. GlideScope Core QuickConnect Cable (0600‑0767) SPECIFICATION VALUE Length (A) 1524 ± 50 mm Diameter (B) 6.8 mm Table 8. BFlex Holder (0810‑0293) SPECIFICATION VALUE Width (A) 220 mm (8.7 in) Length (B) 281 mm (11.1 in) Height (C) 182 mm (7.2 in) Weight... - Page 34 Table 9. BFlex 2 Ultraslim 2.8 (0570‑0434) SPECIFICATION VALUE Length of flexible insertion tube from distal tip (A) 610 mm Outside diameter of flexible insertion tube (B) 2.8 mm Maximum outside diameter of flexible insertion tube and distal tip (C) 3.3 mm...
- Page 35 Table 10. BFlex 2 Slim 3.8 (0570‑0432) SPECIFICATION VALUE Length of flexible insertion tube from distal tip (A) 610 mm Outside diameter of flexible insertion tube (B) 3.8 mm Maximum outside diameter of flexible insertion tube and distal tip (C) 4.4 mm...
- Page 36 Table 11. BFlex 2 Regular 5.0 (0570‑0423) SPECIFICATION VALUE Length of flexible insertion tube from distal tip (A) 610 mm Outside diameter of flexible insertion tube (B) 5.0 mm Maximum outside diameter of flexible insertion tube and distal tip (C) 5.5 mm...
- Page 37 Table 12. BFlex 2 Large 5.8 (0570‑0424) SPECIFICATION VALUE Length of flexible insertion tube from distal tip (A) 610 mm Outside diameter of flexible insertion tube (B) 5.8 mm Maximum outside diameter of flexible insertion tube and distal tip (C) 6.35 mm...
-
Page 38: Electromagnetic Compatibility
Electromagnetic Compatibility The BFlex 2 system is designed to be in compliance with IEC 60601‑1‑2, which contains electromagnetic compatibility (EMC) requirements for medical electrical equipment. The limits for emissions and immunity specified in this standard are designed to provide reasonable protection against harmful interference in a typical medical installation. - Page 39 Electromagnetic immunity Table 14. Guidance and Manufacturer’s Declaration —Electromagnetic Immunity The system is intended for use in the electromagnetic environment specified below. The customer or the user of the system should ensure that it is used in such an environment. COMPLIANCE ELECTROMAGNETIC IMMUNITY TESTS...
- Page 40 Accessory Conformance to Standards To maintain electromagnetic interference (EMI) within certified limits, the system must be used with the cables, components, and accessories specified or supplied by Verathon. For additional information, see the Parts & Accessories section on page 10 and Component Specifications section on page 27.
-
Page 41: Glossary
The following table provides definitions for specialized terms used in this manual or on the product itself. For a full list of caution, warning, and informational symbols used on this and other Verathon products, please refer to the Verathon Symbol Directory at verathon.com/service‑and‑support/symbols. - Page 42 TERM DEFINITION Megahertz Millimeter mmHg Millimeters of mercury non‑powered accessory Endoscopic tool that does not require its own source of electrical power OSHA Occupational Safety and Health Administration (federal agency in U.S.) powered accessory Endoscopic tool that requires its own source of electrical power Radio frequency Relative humidity Sodium dodecyl sulphate...















Need help?
Do you have a question about the BFlex 2 and is the answer not in the manual?
Questions and answers