Table of Contents
Advertisement
Quick Links
Instructions for use
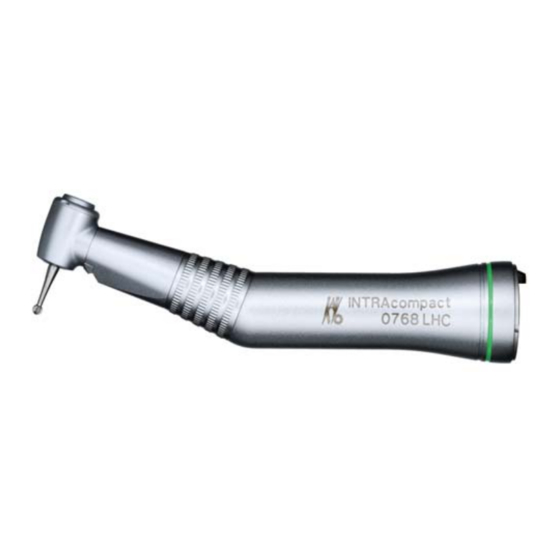
INTRAcompact contra-angle 0768 LHC - REF
1.004.7783
D D istributed by:
KaVo Dental GmbH
Bismarckring 39
D-88400 Biberach
Tel. +49 7351 56-0
Fax +49 7351 56-1488
Contents
C C ontents
1 User instructions ............................................................................................................................................... 5
2 Safety ............................................................................................................................................................... 7
2.1
Description of safety instructions ............................................................................................................ 7
2.1.1
Description of safety instructions: Warning symbol ................................................................... 7
2.1.2
Description of safety instructions: Structure .............................................................................. 7
2.1.3
Description of safety instructions: Description of danger levels ................................................ 8
2.2
Safety instructions .................................................................................................................................. 9
3 Product description ......................................................................................................................................... 16
3.1
Purpose - Proper use .......................................................................................................................... 17
3.2
Technical Data ..................................................................................................................................... 19
3.3
Transportation and storage conditions ................................................................................................. 20
Contents
4 4 First use .......................................................................................................................................................... 22
4.1
Check the amount of water .................................................................................................................. 24
5 Operation ........................................................................................................................................................ 25
5.1
Attaching the medical device ................................................................................................................ 25
5.2
Removing the medical device .............................................................................................................. 27
5.3
Inserting the milling cutters or diamond grinders .................................................................................. 28
5.4
Removing the milling tool or diamond grinder ...................................................................................... 31
6 Troubleshooting .............................................................................................................................................. 34
6.1
Check for malfunctions ......................................................................................................................... 34
6.2
Troubleshooting .................................................................................................................................... 35
6.2.1
Troubleshooting: Exchanging the O-rings on the motor coupling ........................................... 35
6.2.2
Troubleshooting: Cleaning the spray nozzle ........................................................................... 37
7 Setup methods according to DIN EN ISO 17664 ........................................................................................... 38
7.1
Preparation at the site of use ............................................................................................................... 38
Manufacturer:
Kaltenbach & Voigt GmbH
Bismarckring 39
D-88400 Biberach
www.kavo.com
1
2
Advertisement
Table of Contents
Troubleshooting

Summary of Contents for KaVo INTRAcompact contra-angle 0768 LHC
-
Page 1: Table Of Contents
Instructions for use INTRAcompact contra-angle 0768 LHC - REF 1.004.7783 D D istributed by: Manufacturer: KaVo Dental GmbH Kaltenbach & Voigt GmbH Bismarckring 39 Bismarckring 39 D-88400 Biberach D-88400 Biberach Tel. +49 7351 56-0 www.kavo.com Fax +49 7351 56-1488 Contents C C ontents 1 User instructions ............................... - Page 2 Disinfection: Machine disinfection - external and internal ............47 Drying ..............................48 Care products and systems - Servicing ....................49 7.5.1 Care products and systems - Servicing: Care with KaVo Spray ..........50 7.5.2 Care products and systems - Servicing: Care with KaVo SPRAYrotor ........52 7.5.3 Care products and systems - Servicing: Servicing with KaVo QUATTROcare .......
-
Page 3: Safety
Safety 2 2 S S afety 2.1.1 D D escription of safety instructions: Warning symbol Warning symbol 2.1.2 D D escription of safety instructions: Structure DANGER The introduction describes the type and source of the hazard. This section describes the potential consequences of non-observance. ▶... - Page 4 To ensure proper function, the medical device must be set up according to the reprocessing methods described in the KaVo Instructions for Use, and the care products and care systems described therein must be used. KaVo recommends specifying a service interval at the dental office for a licensed shop to clean, service and check the functioning of the medical device.
- Page 5 Safety The following individuals are authorized to repair and service KaVo prod‐ ucts: ▪ Technicians at KaVo branches throughout the world ▪ Technicians specially trained by KaVo Product description 3 3 P P roduct description INTRAcompact contra-angle handpiece 0768 LHC, M M at. no. 1.004.7783 Product description 3 3 .1 P P urpose –...
-
Page 6: Product Description
Product description 3 3 .2 T T echnical Data Drive speed max. 40,000 rpm Transmission ratio 2.7 : 1 1 green ring Pushbutton chuck 2.35 dia. Contra-angle cutters can be used according to DIN EN ISO 1797-1 type 1 The contra-angle handpiece can be mounted on all INTRAmatic Lux mo‐ tors, and motors with a connection in accordance with ISO 3964 / DIN 13940. -
Page 7: Check The Amount Of Water
First use CAUTION Damage from soiled and moist cooling air. Contaminated and moist cooling air can cause malfunctions and lead to premature bearing wear. ▶ Make sure that the supply of cooling air is dry, clean and unconta‐ minated according to ISO 7494-2. First use 4 4 .1 C C heck the amount of water CAUTION... -
Page 8: Removing The Medical Device
Operation Slightly wet the O-rings on the motor coupling with KAVOspray. Place the medical device on the motor coupling and turn it until the catch audibly locks in place. 5.2 R R emoving the medical device Remove the medical device from the motor coupling in an axial direction. Operation 5 5 .3 I I nserting the milling cutters or diamond grinders Note... -
Page 9: Removing The Milling Tool Or Diamond Grinder
Operation ▶ Insert the tool into the segment of the head drive by twisting the tool slightly, and push to the stop. ▶ Check that the tool is seated securely by pulling on it. 5 5 .4 R R emoving the milling tool or diamond grinder CAUTION Hazard from rotating tools. -
Page 10: Troubleshooting: Exchanging The O-Rings On The Motor Coupling
▶ Do not use Vaseline or other grease or oil. Troubleshooting Note The O-rings on the motor coupling may only be lubricated with a cotton ball wetted with KaVo spray. ▶ Press the O-ring between your fingers to form a loop. ▶... -
Page 11: Cleaning
Can only be done with KaVo CLEANspray or KaVo DRYspray. ▶ Cover the medical device with the KaVo CLEANpac bag, and place it on the corresponding care adapter. Press the spray button three times for 2 seconds each time. Remove the medical device from the spray attachment and let the cleaner work for one minute. -
Page 12: Cleaning: Automated Internal Cleaning
The efficacy of manual internal disinfection must be demonstrated by the manufacturer of the disinfection agent. With KaVo products, use only dis‐ infection agents that have been released by KaVo with respect to the com‐ patibility of materials (e.g. WL-cid / made by ALPRO). -
Page 13: Disinfection: Machine Disinfection - External And Internal
7.5.1 C C are products and systems - Servicing: Care with KaVo Spray KaVo recommends servicing the product after each time it is used, i.e. after each automatic cleaning and before each sterilisation. -
Page 14: Care Products And Systems - Servicing: Care With Kavo Sprayrotor
"Care with KaVo Spray". Setup methods according to DIN EN ISO 17664 7 7 .5.2 C C are products and systems - Servicing: Care with KaVo SPRAYrotor KaVo recommends servicing the product after each time it is used, i.e. after each automatic cleaning and before each sterilisation. - Page 15 ISO 17665-1 (e.g. KaVo STERIclave B 2200 / 2200 P) CAUTION Premature wear and malfunctions from improper servicing and care. Reduced product life. ▶ Before each sterilisation cycle, service the medical device with KaVo care products. Setup methods according to DIN EN ISO 17664 CAUTION Contact corrosion due to moisture.
-
Page 16: 8 Auxiliary Equipment
12 months from date of invoice, subject to the following conditions: In case of justified complaints, KaVo will honour its warranty with a free replacement or repair. Other claims of any nature whatsoever, in particular with respect to compensation, are excluded.
















Need help?
Do you have a question about the INTRAcompact contra-angle 0768 LHC and is the answer not in the manual?
Questions and answers