Lowenstein Medical LUISA Instructions For Use Manual
Ventilators
Hide thumbs
Also See for LUISA:
- Instructions manual (52 pages) ,
- Instructions for use manual (40 pages) ,
- Support manual (4 pages)
Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for Lowenstein Medical LUISA
- Page 1 EN Instructions for Use for devices of type LM150TD LUISA Ventilators...
-
Page 2: Table Of Contents
Contents Contents Introduction Settings in the menu 1.1 Intended use ............ 3 5.1 Navigating in the menu ......... 15 1.2 Description of function ........3 5.2 Menu structure for patient menu ....15 1.3 User qualification ..........3 Hygiene treatment and servicing 1.4 Indications ............ -
Page 3: Introduction
USB-C stick and analyzed by PC software. 1.1 Intended use 1.3 User qualification The LM150TD LUISA ventilator is for the life-support and The person operating the device is referred to in these non-life-support ventilation of patients who require instructions for use as the user. -
Page 4: Side Effects
2 Safety Only use accessory parts from the manufacturer. Relative contraindications: Do not use antistatic or electrically-conductive tubes. Cardiac decompensation, severe cardiac arrhythmias, severe The accuracy of the device may be impaired by the gas hypotension, especially in combination with intravascular supplied by a pneumatic nebulizer. -
Page 5: General Information
Water and dirt in 2.3 Safety information in the device may damage the device. Only operate the device in the associated LUISA mobility these instructions for use bag. Transport or store the device in the associated LUISA protective bag. -
Page 6: Product Description
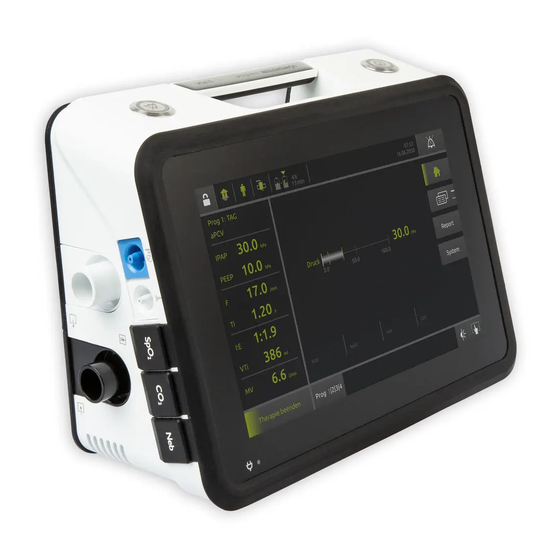
3 Product description 3 Product description 3.1 Overview 2 3 4 10 11 External battery connection Filter compartment with coarse dust filter and fine filter Connection for monitor/prismaHUB Compartment for internal battery USB-C connection Exhalation tube connection Nurse call system connection Device outlet port Power supply indicator Handle... -
Page 7: Control Panel In Display
3 Product description 3.2 Control panel in display Status line - symbols indicate current device status (e.g. accessories connected, battery capacity). Alarm acknowledgement key - Press briefly: Acknowledges alarm. If the alarm persists, the alarm is muted for 120 seconds. Press and hold: Mutes all acoustic alarms for 2 minutes. -
Page 8: Symbols In Display
Gray: Not connected Green: Connected and high signal quality Protective bag for Serves to transport and store the Yellow: Connected and moderate signal LUISA device with protection quality Red: Connected and poor signal quality Follow the instructions for use for the accessories. -
Page 9: Operating States
3 Product description 3.5 Operating states 3.6.2 External batteries • External batteries can be connected to the device as an • On: Therapy is in progress. It is possible to make device additional energy supply. If the device is connected to and therapy settings. -
Page 10: Data Management/Compatibility
The device configuration is retained following the update. 3.7.3 Setting up a connection to the LUISA app The LUISA app is an app on a mobile terminal. The device can be connected to the LUISA app. In the System menu, check whether the Bluetooth®... -
Page 11: Preparation And Operation
4 Preparation and operation 4 Preparation and operation 4.1 Setting up and connecting device Risk of injury from inadequate therapy if air inlet and 2. Connect the power cable to the power supply unit and air outlet are blocked! the socket. A blocked air inlet and/or air outlet can cause the device to overheat, impair therapy and damage the device. - Page 12 4 Preparation and operation 4.2.1 Connecting leakage circuit 4.2.3 Connecting double circuit 1. Push the free end of the breathing tube 1 onto the 1. Push the breathing tube onto the device outlet port. device outlet port. 2. Connect the invasive or non-invasive patient/ventilator 2.
-
Page 13: Before First Use
4 Preparation and operation 4.2.5 Connecting the circuit for Alternatively In battery mode: Press and hold On/off key HFT mode approx. 1 second. The device automatically performs a few function tests. The alarm system is tested automatically. If the device is fully functional, the home screen is displayed and the device switches to standby. -
Page 14: Performing Tube Test
4 Preparation and operation 4.7 Performing tube test Perform a tube test at every function check, on change of patient and as required. This checks for resistance, compliance and leaks. Requirement Type of tube used is selected in the Ventilation menu. 1. -
Page 15: Settings In The Menu
5 Settings in the menu 5.1 Navigating in the menu ACTION FUNCTION ACTION FUNCTION Opens range of values for setting Function keys have a gray Press on value ventilation parameters background and the function is displayed on the key in text or as Move range of values Decrease or increase value a symbol (e.g. - Page 16 5.2.2 Report menu in the patient menu 5.2.4 Device settings submenu (usage data) PARAMETER DESCRIPTION Information about the parameters in this menu can be found in the table below. The patient can set the alarm level here. 1= very quiet, 2= quiet, 3= loud, 4= Alarm volume PARAMETER DESCRIPTION...
-
Page 17: Hygiene Treatment And Servicing
6 Hygiene treatment and servicing 6.1 Hygiene treatment 6.1.2 Cleaning intervals INTERVAL ACTION Clean device (see "6.1.3 Subjecting device Weekly to a hygiene treatment", page 17). Clean coarse dust filter (see " Cleaning Risk of infection when the device is used again! coarse dust filter (gray filter)", page 18). - Page 18 Cleaning coarse dust filter (gray filter) Cleaning exhalation module 1. Open filter compartment flap. 2. Remove gray coarse dust filter. 3. Wash coarse dust filter under running water. 1. To open the exhalation module compartment on the rear of the device, turn the latch counterclockwise to the 4.
-
Page 19: Function Check
Cleaning filter for cooling air fan 10. Check the functionality of the batteries: • Disconnect the device from the power supply. 1. Open exhalation module compartment (see " Cleaning exhalation module", page 18). The first external battery (if present) takes over energy supply (watch what is shown in display). -
Page 20: Checking Alarms
6.3 Checking alarms 6.3.1 Non-specialist user (patient or relatives) ALARM ID NO. REQUIREMENT TEST On a single circuit with valve: Alarm limit is set to a value <150 l/m With leakage ventilation: Leave breathing tube open at patient connection. Leakage high Alarm limit is set to a value <60 l/m Start ventilation. -
Page 21: Alarms
7 Alarms 7 Alarms 7.3 Configuring physiological alarms A distinction is made between two types of alarm: Physiological alarms relate to ventilation of the patient. All physiological alarms are deactivated on delivery or when Technical alarms relate to configuration of the device. the device is reset to factory settings. - Page 22 7 Alarms DISPLAY CODE CAUSE ACTION Check connection from device to patient/ Leakage high ventilator interface at the patient via the Leak breathing tube. Check that the patient/ventilator interface is in position correctly. Minute volume high Maximum minute volume exceeded. Check therapy and alarm settings.
-
Page 23: Technical Alarms
7 Alarms 7.4 Technical alarms DISPLAY CODE CAUSE ACTION Service necessary. Please Technical fault which can only be get in touch with your Various eliminated by an authorized specialist Have device repaired. specialist dealer/contact. dealer. Intake air temperature high Operate device at an ambient temperature Ambient temperature too high. - Page 24 7 Alarms DISPLAY CODE CAUSE ACTION Temperature of internal Battery will switch off due to temperature. battery critically high Internal battery too warm. Operate device at an ambient temperature of 5 °C to 40 °C. Internal battery Battery has switched off due to temperature. overheated Internal battery overheated.
- Page 25 7 Alarms DISPLAY CODE CAUSE ACTION Temperature of battery E2 low Operate device at an ambient temperature External battery 1 too cold. of 5 °C to 40 °C. Internal battery communication fault Internal battery defective. Contact your specialist dealer. Device defective. Battery E1 communication fault External battery 1 defective.
- Page 26 7 Alarms DISPLAY CODE CAUSE ACTION Constant pressure level Respiratory frequency or set pressure Check therapy settings. difference too low. Intake area blocked Intake area blocked. Keep intake area free. Valve control tube and pressure Check circuit and tube connections are Pressure measuring tube measuring tube switched.
-
Page 27: Nurse Call And Remote Alarm
7 Alarms DISPLAY CODE CAUSE ACTION Blower overheated Therapy will end. Blower has overheated. Allow device to cool down. Maximum device pressure Reduce resistance and restart device. exceeded Resistance on inspiration too high. If alarm recurs: Contact your specialist dealer. Maximum device pressure Reduce resistance and restart device. -
Page 28: Technical Specifications
9 Technical specifications SPECIFICATION DEVICE Product class to 93/42/EEC Dimensions W x H x D in cm 30 x 13 x 21 Weight 3.8 kg Temperature range - Operation +5 °C to +40 °C -25 °C to +70 °C - Transport and storage Allow to cool to room temperature for 4 hours before - Transport and storage at +70 °C starting up. - Page 29 SPECIFICATION DEVICE Internal/external battery Li-ion Type 3200 mAh Nominal capacity 29.3 V Nominal voltage 93.7 Wh Energy 500 charging cycles Typical discharge cycles Battery capacity is reduced when the device is operated at low temperatures. Operating hours of internal battery assuming following settings: Double circuit, PCV mode, f=20/min, Ti=1 s, ...
- Page 30 SPECIFICATION DEVICE EPAP pressure range 4 hPa - 25 hPa Most disadvantageous circuit for leakage ventilation: Breathing tube WM 29988, bacteria filter WM 27591 0 hPa - 25 hPa PEEP pressure range Most disadvantageous circuit for valve ventilation: Breathing tube LMT31383, bacteria filter WM 27591 Accuracy of airway pressure ±(2 hPa + 4 % of the set value) ±(2 cmH2O + 4 % of the set value)
- Page 31 SPECIFICATION DEVICE Speed of pressure reduction (in leakage ventilation only) Level 1: -100 hPa/s Adult Level 2: -80 hPa/s Level 3: -50 hPa/s Level 4: -20 hPa/s Level 1: -135 hPa/s Child Level 2: -100 hPa/s Level 3: -80 hPa/s Level 4: -50 hPa/s Maximum permitted flow for oxygen supply 30 l/min...
- Page 32 The software of this device contains code which is subject to the GPL. You can obtain the source code and the GPL on request.
-
Page 33: Annex
10 Annex 10 Annex 10.1 Pneumatic diagram 10.1.1 Leakage ventilation Leakage Circuit O2 pathway O2 supply Patient Interface inspiration Pressure sensor for patient pressure exhalation Spontaneous ambient air breathing & Optional Optional: Optional: Air filter Blower Flow sensor Patient circuit Leakage system non-return O2- sensor... -
Page 34: System Resistances
ARTICLE NAME NUMBER IN L/MIN IN HPA LMT 31382 LUISA, single circuit with valve, 180 cm, 22 mm Ø 0.11 LMT 31383 LUISA, single circuit with valve, 150 cm, 15 mm Ø 0.46 LUISA, single circuit with valve, heated (i), autofill LMT 31384 2.04... -
Page 35: Emission Of Electromagnetic Interference
PRESSURE REDUCTION ARTICLE NAME NUMBER IN L/MIN IN HPA Inspiration tube: 2.03 LUISA, double circuit, heated (i+e), A-adapter, autofill Inspiration tube from patient LMT 31582 chamber, 150 cm + 60 cm, 15 mm Ø to device: 2.05 Exhalation tube: 2.06 Inspiration tube: 0.22... -
Page 36: Markings And Symbols
10.6 Scope of delivery Order number 10.6.1 Scope of delivery for LMT 31380- Suitable for use in aircraft. Meets 1110 LUISA with HFT mode RTCA/DO-160G Section 21, Category M. The parts below are included in the standard scope of delivery:... -
Page 37: Accessories
Instructions for use LMT 68651 VENTIremote alarm, 30 m LMT 31570 Final inspection log LMT 31588 Cable, 10 m, nurse call for LUISA LMT 31510 Accessories bag LMT 31440 Cable, 30 m, nurse call for LUISA LMT 31520 CD-ROM with prismaTS software WM 93331 10.7 Accessories... -
Page 38: Warranty
10 Annex 10.9 Warranty Löwenstein Medical Technology gives the purchaser of a new original product and of a spare part fitted by Löwenstein Medical Technology a limited manufacturer warranty in accordance with the warranty conditions applicable to the product in question and in accordance with the warranty periods from date of purchase listed below. - Page 40 Löwenstein Medical Technology GmbH + Co. KG Kronsaalsweg 40 22525 Hamburg, Germany T: +49 40 54702-0 F: +49 40 54702-461 www.loewensteinmedical.de...












Need help?
Do you have a question about the LUISA and is the answer not in the manual?
Questions and answers
who reads report machine has a reader plug in device is that something we take to a Doctor office ? Is our report monitored as we sleep?
The reader plug-in device, specifically the USB flash drive (LMT 31414), is used to transmit therapy data from the LUISA device in the form of EDF files. These files can then be read and displayed in the prismaTS software.
There is no information in the provided context confirming that the sleep report is monitored by a doctor.
This answer is automatically generated