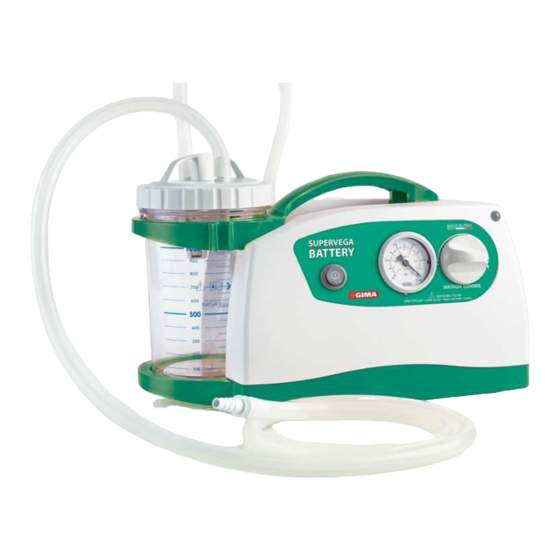
Gima SUPER VEGA BATTERY Instruction Manual
Hide thumbs
Also See for SUPER VEGA BATTERY:
- Manual (120 pages) ,
- Instruction manual (33 pages) ,
- Manual (13 pages)
Table of Contents
Advertisement
Quick Links
28243
M28243 - M-Rev.1-29.11.16
GIMA S.p.A. - Via Marconi, 1 - 20060 Gessate (MI) - Italy
ITALIA: Tel. 02 953854.1 – Fax. 02 95381167 / e-mail:
INTERNATIONAL: Tel. +39 02 953854209/221/225 – Fax. +39 02 95380056
e-mail:
export@gimaitaly.com
ASPIRATORE SUPER VEGA BATTERY
SUPER VEGA BATTERY ASPIRATOR
ASPIRATEUR SUPER VEGA BATTERY
ABSAUGER SUPER VEGA BATTERY
ASPIRADOR SUPER VEGA BATTERY
ASPIRADOR SUPER VEGA BATTERY
Manuale d'uso
Instruction manual
Mode d'emploi
Manual de istrucciones
Manual de instruções
gima@gimaitaly.com
–
www.gimaitaly.com
Handbuch
–
www.gimaitaly.com
1
Advertisement
Table of Contents

Summary of Contents for Gima SUPER VEGA BATTERY
-
Page 1: Condizioni Di Garanzia
Mode d'emploi Handbuch Manual de istrucciones Manual de instruções M28243 - M-Rev.1-29.11.16 GIMA S.p.A. - Via Marconi, 1 - 20060 Gessate (MI) - Italy ITALIA: Tel. 02 953854.1 – Fax. 02 95381167 / e-mail: gima@gimaitaly.com – www.gimaitaly.com INTERNATIONAL: Tel. +39 02 953854209/221/225 – Fax. +39 02 95380056 e-mail: export@gimaitaly.com... -
Page 2: Table Of Contents
AVVERTENZE ............................................4 NORME DI SICUREZZA FONDAMENTALI ................................4-5 CARATTERISTICHE TECNICHE ....................................6 PULIZIA DELL’UNITÀ PRINCIPALE ................................... 6 SIMBOLOGIA ............................................7 ACCESSORI IN DOTAZIONE ......................................8 PULIZIA ACCESSORI ........................................8-9 CONTROLLO PERIODICO DI MANUTENZIONE ............................. 9-10 ISTRUZIONI PER L’USO ......................................10-12 CONDIZIONI DI GARANZIA ......................................12 MODALITÀ... - Page 3 ПРЕДУПРЕЖДЕНИЯ ........................................66 ОСНОВНЫЕ ПРАВИЛА ТЕХНИКИ БЕЗОПАСНОСТИ ..........................66-67 ТЕХНИЧЕСКИЕ ХАРАКТЕРИСТИКИ ................................... 68 ОЧИСТКА ГЛАВНОГО БЛОКА ....................................69 СИСТЕМА УСЛОВНЫХ ОБОЗНАЧЕНИЙ ................................69 КОМПЛЕКТУЮЩИЕ В ОСНАЩЕНИИ ................................70 ОЧИСТКА КОМПЛЕКТУЮЩИХ ..................................70-71 ПЕРИОДИЧЕСКИЕ ПРОВЕРКИ И ТЕХОБСЛУЖИВАНИЕ ........................71-72 ИНСТРУКЦИИ ПО ЭКСПЛУАТАЦИИ ................................72-74 УСЛОВИЯ...
-
Page 4: General Warning
Instrument and accessory discharging must be done according to current regulations in the country of use. WARNING: Do not change this equipment without the permission of the manufacturer GIMA S.p.A. None of electric or mechanical parts have been designed to be repaired by customers or end-users. Don’t open the device, do not mishandle the... -
Page 5: Technical Characteristics
Using the device in environmental conditions different than those indicated in this manual may harm seriously the safety and the technical characteristics of the same. The medical device is in contact with the patient by means of a disposable probe (not supplied with the device). Suction tubes for insertion in the human body purchased separately from the machine should comply with ISO 10993-1 standards on material biocompatibility. -
Page 6: Symbols
CE marking in conformity with EC directive 93/42/CEE and subsequent changes General warnings and/or specifications Consult the instruction manual Manufacturer: GIMA S.p.A. - Via Marconi, 1 - 20060 Gessate (MI) - Italy Date of manufacture Applied Part type BF (suction probe) Keep in a cool, dry place Conservation temperature: - 25 ÷... -
Page 7: Accessories Supplies
Check the expiry date on the original packaging of the suction catheter and check the integrity of the sterile packaging. GIMA declines any liability for injury to the patient correlated to the deterioration of the above-mentioned sterile packaging due to handling of the original packaging by third parties. -
Page 8: Periodical Maintenance Checks
Contcat the technical service Faults 1 - 2 - 3 - 4 - 5 - 6 - 7 - 8 – None of the remedies has Contact the seller or GIMA After-sales Assistance 9 - 10 achieved the desired results... -
Page 9: Instruction For Use
2° case – If both the security system doesn’t work, there is the possibility that liquid comes inside the device, in this case return the device to GIMA technical service. GIMA S.p.A. will provide upon request electric diagrams, components list, descriptions, setting instructions and any other information that can help the technical assistance staff for product repair. - Page 10 WARNING: Ensure that the FLUID SIDE or IN marker on the filter is on the side facing the collection jar lid and fitted into the “VACUUM”. A wrong connection causes immediate destruction in case of contact with sucked liquids. Filter assembling FLOW DIRECTION IN / Fluid Inside Suction Pump...
-
Page 11: Risk Of Electromagnetic Interference And Possible Remedies
NEVER USE THE DEVICE WITHOUT JAR AND / OR PROTECTION FILTER RISK OF ELECTROMAGNETIC INTERFERENCE AND POSSIBLE REMEDIES This section contains information regarding the conformity of the compliance with the EN 60601-1-2 Standard. The SUPERVEGA BATTERY 230/12V surgical aspirator is an electro-medical device that requires particular precautions regarding electro-magnetic compatibility and which must be installed and commissioned according to the electro-magnetic compatibility information supplied. - Page 12 Guidance and manufacturer’s declaration – Immunity Emissions The surgical aspirator SUPERVEGA BATTERY 230/12V is intended for use in the electromagnetic environment specified below. The customers or the user of the surgical aspirator SUPERVEGA BATTERY 230/12V should assure that it’s used in such an environment.
-
Page 13: Warranty Conditions
Failure to follow this procedure will result in the instrument being returned to the purchaser unrepaired. Instruments returned for repair MUST be accompanied by a description of the problem. GIMA will not be responsible for damage caused through improper use. To avoid such damage, please read the instruction carefully. -
Page 15: Caractéristiques Techniques
Via Marconi, 1 – 20060 Gessate (MI) – Italia ITALIA: Tel. 02 953854.1 – Fax. 02 95381167 e-mail: gima@gimaitaly.com – www.gimaitaly.com INTERNATIONAL: Tel. +39 02 953854209/221/225 – Fax. +39 02 95380056 e-mail: export@gimaitaly.com – www.gimaitaly.com GIMA S.p.A. - Via Marconi, 1 - 20060 Gessate (MI) - Italy...














Need help?
Do you have a question about the SUPER VEGA BATTERY and is the answer not in the manual?
Questions and answers