Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents
Troubleshooting

Summary of Contents for Mindray BeneHeart D30
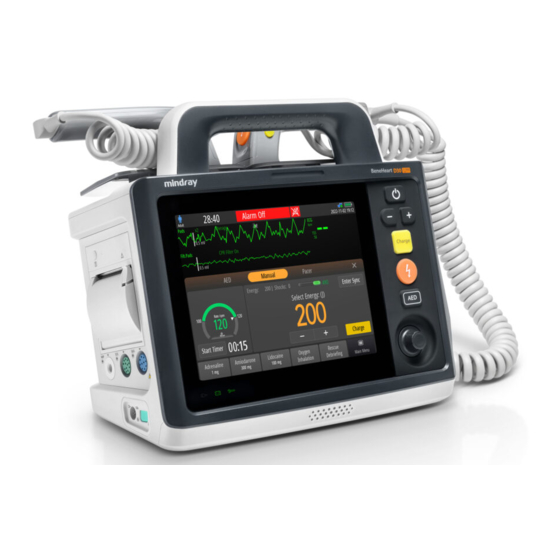
- Page 1 BeneHeart D30/BeneHeart D20A BeneHeart D20/BeneHeart D20C Defibrillator/Monitor...
- Page 3 2797 © Copyright 2022 Shenzhen Mindray Bio-Medical Electronics Co., Ltd. All rights reserved. Release time: June 2022 Revision: 1.0...
- Page 4 SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS CO., LTD. (hereinafter called Mindray) owns the intellectual property rights to this Mindray product and this manual. This manual may refer to information protected by copyrights or patents and does not convey any license under the patent rights of Mindray, nor the rights of others.
- Page 5 Mindray's obligation or liability under this warranty does not include any transportation or other charges or liability for direct, indirect or consequential damages or delay resulting from the improper use or application of the product or the use of parts or accessories not approved by Mindray or repairs by people other than Mindray authorized personnel.
- Page 6 Notification of Adverse Events As a health care provider, you may report the occurrence of certain events to SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS CO., LTD., and possibly to the competent authority of the Member state in which the user and / or patient is established.
-
Page 7: Table Of Contents
Contents 1 Safety ....................................1 - 1 1.1 Safety Information ......................................1 - 1 1.1.1 Dangers ........................................1 - 1 1.1.2 Warnings ........................................1 - 1 1.1.3 Cautions ........................................1 - 2 1.1.4 Notes .........................................1 - 3 2 Equipment Overview ................................ 2 - 1 2.1 Intended Use ........................................2 - 1 2.1.1 Intended Purpose Statement ................................2 - 1 2.1.2 Indication for Use ....................................2 - 1... - Page 8 7.2 CPR Metronome .........................................7 - 1 7.2.1 Viewing Filtered ECG Waveform ..............................7 - 1 7.3 CPR Feedbacks ........................................7 - 2 7.4 CPR Quality (CQI) Monitoring ..................................7 - 2 8 Noninvasive Pacing ................................8 - 1 8.1 Pacing Safety Information ....................................8 - 1 8.2 Choosing the Pacer Mode Setting ................................8 - 2 8.2.1 Demand Mode Pacing Procedure ..............................8 - 2 8.2.2 Fixed Mode Pacing Procedure ................................8 - 2...
- Page 9 11.5.2 Changing Arrhythmia Settings ..............................11 - 4 11.6 ST Segment Monitoring ....................................11 - 5 11.6.1 ST Safety Information ..................................11 - 5 11.6.2 Saving the Current ST as Baseline ............................. 11 - 5 11.7 QT/QTc Interval Monitoring ..................................11 - 5 11.7.1 Displaying QT/QTc Numerics ..............................
- Page 10 16.2.1 Preparing to Sidestream CO Measurement ........................16 - 1 16.2.2 Zeroing the Sidestream CO Module ............................16 - 1 16.3 Changing CO Settings ....................................16 - 2 16.3.1 Setting the Gas Compensation ..............................16 - 2 16.3.2 Changing Barometric Pressure ..............................16 - 2 16.4 CO Calibration ......................................
- Page 11 24.2.2 Cleaning the Thermal Print Head .............................. 24 - 1 24.3 Cleaning and Disinfecting the Accessories ............................24 - 2 25 Maintenance ..................................25 - 1 25.1 Maintenance Safety Information ................................25 - 1 25.2 Routine Maintenance ....................................25 - 1 25.2.1 Auto Test ......................................
- Page 12 This page intentionally left blank.
-
Page 13: Safety
Safety Safety Information DANGER • Indicates an imminent hazard that, if not avoided, will result in death or serious injury. WARNING • Indicates a potential hazard or unsafe practice that, if not avoided, could result in death or serious injury. CAUTION •... -
Page 14: Cautions
• The software equipment copyright is solely owned by Mindray. No organization or individual shall resort to modifying, copying, or exchanging it or to any other infringement on it in any form or by any means without due permission. -
Page 15: Notes
• At the end of its service life, the equipment, as well as its accessories, must be disposed of in compliance with the guidelines regulating the disposal of such products. If you have any questions concerning disposal of the equipment, please contact us. •... - Page 16 This page intentionally left blank. 1 - 4...
-
Page 17: Equipment Overview
Equipment Overview Intended Use 2.1.1 Intended Purpose Statement The equipment is intended for external defibrillation, internal defibrillation, synchronized cardioversion and semi-automated defibrillation (AED). It can also be used for non-invasive external pacing, CPR feedback as well as ECG, Resp, SpO , PR, NIBP and CO monitoring. -
Page 18: Contra-Indications
2.1.6 Contra-indications ■ The AED mode is contraindicated in the treatment when the patient is showing any of the following: ◆ Consciousness ◆ Breathing ◆ Detectable pulse or other signs of circulation ■ Manual Defibrillation Manual defibrillation is contraindicated in the treatment when the patient is showing any of the following: ◆... -
Page 19: Equipment Preparation
Any personnel who connect devices to the equipment’s signal input/output port are responsible for providing evidence that the safety certification of the devices has been performed in accordance to the IEC 60601-1. If you have any questions, please contact Mindray. •... -
Page 20: Turning On The Equipment
• Do not use the equipment on a patient if you suspect it is not working properly, or if it is mechanically damaged. Contact the service personnel or Mindray. • Check that visual and auditory alarm signals are presented correctly when the equipment is turned... -
Page 21: Therapy Preparation
Therapy Preparation Checking the Patient Contact Indicator NOTE • It is recommended to perform the defibrillation on a patient when the patient contact indicator illuminates in green. If the patient contact indicator illuminates in orange, it also can be used for the defibrillation. - Page 22 This page intentionally left blank. 4 - 2...
-
Page 23: Aed
AED Safety Information DANGER • Defibrillation current can cause operator or bystander severe injury or even death. Do not touch the patient or any metal objects (including bed or gurney) connected to the patient during defibrillation. • Avoid contact between parts of the patient’s body such as exposed skin of head or limbs, conductive fluids such as gel, blood, or saline, and metal objects such as a bed frame or a stretcher which may provide unwanted pathways for the defibrillating current. -
Page 24: Aed Procedure
AED Procedure NOTE • Anterior - lateral placement for adult patients, and anterior-posterior placement for pediatric patients are recommended placements for defibrillation with electrode pads. • For defibrillation of pediatric patients under 8 years, pediatric electrode pads should be used. •... -
Page 25: Manual Defibrillation
Manual Defibrillation Manual Defibrillation Safety Information DANGER • Defibrillation current can cause operator or bystander severe injury or even death. Do not touch the patient or any metal objects (including bed or gurney) connected to the patient during defibrillation. • Avoid contact between parts of the patient’s body such as exposed skin of head or limbs, conductive fluids such as gel, blood, or saline, and metal objects such as a bed frame or a stretcher which may provide unwanted pathways for the defibrillating current. -
Page 26: External Defibrillation Procedure
• Successful resuscitation is dependent on many variables specific to the patient’s physiological state and the circumstances surrounding the patient event. Failure to have a successful patient outcome is not a reliable indicator of equipment performance. The presence or absence of a muscular response to the transfer of energy during electrical therapy is not a reliable indicator of energy delivery or equipment performance. -
Page 27: Synchronized Cardioversion Procedure
6.4.1 Synchronized Cardioversion Procedure NOTE • During synchronized cardioversion, the shock will be delivered when the equipment detects the next R-wave. If electrode electrodes or internal paddles without button are used, you should press and hold the Shock button on the equipment until the shock is delivered. If external paddles are used, you should press and hold the Shock buttons on both the external paddles until the shock is delivered. - Page 28 This page intentionally left blank. 6 - 4...
-
Page 29: Cpr Assistance
CPR Assistance CPR Assistance Safety Information WARNING • Perform CPR on a patient on firm ground if possible. When you perform CPR on a patient lying on a mattress, a backboard must be used to limit the amount of compressed depth which is absorbed by the mattress. -
Page 30: Cpr Feedbacks
CPR Feedbacks NOTE • When the interruption time exceeds five minutes, a CPR event is automatically generated. CPR Quality (CQI) Monitoring WARNING • CQI monitoring is not intended for pediatric and neonatal patients. • CQI results should not be used as the sole basis for diagnosis or therapy decisions. It is not intended to replace the competent judgment of a clinician. -
Page 31: Noninvasive Pacing
Noninvasive Pacing Pacing Safety Information WARNING • Heart rate and related alarms may be unreliable during pacing, you should always keep the patient under close survillance. The indicated heart rate or related alarms cannot be used as the sole basis for the patient’s perfusion status. -
Page 32: Choosing The Pacer Mode Setting
Choosing the Pacer Mode Setting NOTE • Use the demand mode pacing whenever possible. Only use the fixed mode pacing when there are interferes causing R-wave unreliable or no available ECG electrodes. 8.2.1 Demand Mode Pacing Procedure CAUTION • Routinely assess the patient’s cardiac output. NOTE •... -
Page 33: Monitoring Preparation
Monitoring Preparation Defining the Monitoring Display 9.1.1 Setting the Switch for a Parameter NOTE • When a parameter is manually switched off, you cannot monitor this parameter even if related accessories are connected. 9.1.2 Defining the Normal Screen Display NOTE •... - Page 34 This page intentionally left blank. 9 - 2...
-
Page 35: Alarms
Alarms 10.1 Alarm Introduction NOTE • When multiple alarms of different priority levels occur simultaneously, the equipment selects the alarm of the highest priority to light the alarm lamp and issue the alarm tone. • When multiple alarms of different priority levels occur simultaneously and should be displayed in the same area, the equipment only displays the messages of the highest priority alarm. -
Page 36: Setting Alarm Tone Properties
10.2.1 Setting Alarm Tone Properties NOTE • When the alarm volume is set to 0, the alarm sound is turned off and the audio off symbol is displayed in the alarm status area. • You cannot set the volume of high priority alarms if Alarm Volume is set to 0. 10.2.2 Setting the Switch of SpO Desat Alarm Off... -
Page 37: Switching Off Alarm Sounds
10.6 Switching Off Alarm Sounds WARNING • Switching off alarm sounds may result in a hazard to the patient. 10.7 Resetting Alarms NOTE • If a new alarm is triggered after the alarm system is reset, the alarm reset symbol will disappear, indications of the alarm lamp, alarm tone and alarm messages will be reactivated. - Page 38 This page intentionally left blank. 10 - 4...
-
Page 39: Monitoring Ecg
Monitoring ECG 11.1 ECG Safety Information WARNING • ECG monitoring provided by this equipment is not intended for direct cardiac application. • Make sure the conductive parts of electrodes and associated connectors, including the neutral electrode, do not contact any other conductive parts including earth. •... -
Page 40: Applying Ecg Electrodes
11.3.2 Applying ECG Electrodes NOTE • Store the electrodes at room temperature. • Only open the electrode package immediately prior to use. • Never mix patient electrode types or brands. This may lead to problem due to impedance mismatch. • When applying the electrodes, avoid bony area, obvious layers of fat, and major muscles. -
Page 41: Changing Ecg Settings
11.4 Changing ECG Settings 11.4.1 Setting the Analysis Mode NOTE • It is difficult for the equipment to differentiate an aberrantly conducted beat from a ventricular beat. An aberrantly conducted beat may be misclassified as a ventricular beat. In this case, choose the lead with a narrow R-wave for ECG1 and select Single Lead. -
Page 42: Changing Arrhythmia Settings
CAUTION • Since the arrhythmia detection algorithm sensitivity and specificity are less than 100%, sometimes there may be some false arrhythmias detected and also some true arrhythmia events may not be detected. This is especially true when the signal is noisy. •... -
Page 43: St Segment Monitoring
11.5.2.5 Setting Arrhythmia Refractory Periods NOTE • Refractory periods are only applicable to arrhythmias in the medium priority chains. • Refractory periods have no impact on Tachy, Brady, HR High, HR Low, A-Fib/A-Fib End, Irr Rhythm/Irr Rhythm End. 11.6 ST Segment Monitoring 11.6.1 ST Safety Information WARNING... -
Page 44: Ecg Troubleshooting
11.9 ECG Troubleshooting NOTE • For the physiological and technical alarm messages, see D Alarm Messages. 11 - 6... -
Page 45: Resting 12-Lead Ecg Analysis
Resting 12-Lead ECG Analysis 12.1 Resting 12-Lead ECG Analysis Introduction WARNING • Resting 12-lead ECG analysis provided by this equipment is not intended for direct cardiac application. 12.2 Changing 12-Lead ECG Settings NOTE • For patients under 16 years old, it is recommended to V3 Placement to V4R and place chest electrodes at V4R, V1, V2, V4, V5, V6. - Page 46 This page intentionally left blank. 12 - 2...
-
Page 47: Monitoring Respiration (Resp)
Monitoring Respiration (Resp) 13.1 Resp Safety Information WARNING • When monitoring the patient’s respiration, do not use ESU-proof ECG cables. • If you do not set the detection level for the respiration correctly in manual detection mode, it may not be possible for the equipment to detect apnea. If you set the detection level too low, the equipment is more likely to detect cardiac activity, and to falsely interpret cardiac activity as respiratory activity in the case of apnea. -
Page 48: Changing Resp Settings
• For patients expand chests laterally (normally neonatal patients), to avoid negative intrathoracic pressure and optimize respiratory waveforms, respectively apply the electrodes in the right midaxillary and the left lateral chest areas at the maximum point of the breathing movement. •... -
Page 49: Monitoring Pulse Oxygen Saturation (Spo )
The SpO extension cable should be compatible with the SpO connectors. For example, you can only the Mindray SpO extension cable to the Mindray SpO connectors. • Measurement accuracy verification: The SpO accuracy has been verified in human experiments by comparing with arterial blood sample reference measured with a CO-oximeter. -
Page 50: Spo Display
Refer to the Cable or Sensor DFU for the specified duration of the patient monitoring time. 14.3 Display NOTE • PI is only available for Mindray SpO and Masimo SpO 14.4 Preparing for SpO Monitoring CAUTION •... -
Page 51: Nellcor Sat-Seconds Alarm Management
14.5.2 Nellcor Sat-Seconds Alarm Management NOTE • The SpO Too Low or SpO Too High alarm is presented in the case that SpO value violates the alarm limits for 3 times within one minute even if the setting of Sat-Seconds is not reached. 14.5.3 Setting SpO Sensitivity (for Masimo SpO... - Page 52 This page intentionally left blank. 14 - 4...
-
Page 53: Monitoring Noninvasive Blood Pressure (Nibp)
Monitoring Noninvasive Blood Pressure (NIBP) 15.1 NIBP Introduction NOTE • Blood pressure measurements determined with this device are equivalent to those obtained by a trained observer using the cuff/stethoscope auscultatory method or an intra-arterial blood pressure measurement device, within the limits prescribed by the American National Standard: manual, electronic, or automated sphygmomanometers. -
Page 54: Nibp Measurement Limitations
15.3 NIBP Measurement Limitations NOTE • The effectiveness of the sphygmomanometer has not been established in pregnant, including pre- eclamptic patients. 15.4 NIBP Display NOTE • If NIBP measurement fails, “XX” is displayed; if NIBP measurement is not taken, “--” is displayed. •... -
Page 55: Monitoring Carbon Dioxide (Co )
Monitoring Carbon Dioxide (CO 16.1 Safety Information WARNING • Route all tubing away from the patient’s throat to avoid strangulation. CAUTION • Remove the airway sample line from the patient’s airway while nebulized medications are being delivered. • EtCO values measured from the CO module may differ from those of from the blood gas analysis. -
Page 56: Changing Co Settings
16.3 Changing CO Settings 16.3.1 Setting the Gas Compensation WARNING • Make sure to use the appropriate compensations. Inappropriate compensations may cause inaccurate measurement values and result in misdiagnosis. 16.3.2 Changing Barometric Pressure 16.4 Calibration CAUTION • Connect the gas outlet to the scavenging system when calibrating the CO module. -
Page 57: Clinical Assistive Applications
Clinical Assistive Applications 17.1 Glasgow Coma Scale (GCS) CAUTION • GCS is intended as an adjunct in patient assessment and must be used in conjunction with observation of clinical signs and symptoms. • GCS is not applied to patients that are sedated, muscularly relaxed, with artificial airway, drunk, or in status epilepsies. -
Page 58: Heart Score
17.3 HEART Score NOTE • A license is required for HEART score. 17.4 Traumatic Brain Injury (TBI) Assessment NOTE • A license is required for TBI assessment. 17 - 2... -
Page 59: Review
Review 18.1 Reviewing Events NOTE • A total loss of power has no impact on the events stored. • Alarms are saved as events and will be maintained if the equipment is powered down. The time of equipment power down is not recorded as an event and cannot be reviewed. •... - Page 60 This page intentionally left blank. 18 - 2...
-
Page 61: Printing
Printing 19.1 Loading Paper CAUTION • Use only specified thermal paper. Otherwise, it may cause damage to the recorder’s printhead, the recorder may be unable to print, or poor print quality may result. • Never pull the recorder paper with force when a recording is in process. Otherwise, it may cause damage to the recorder. - Page 62 This page intentionally left blank. 19 - 2...
-
Page 63: Discharged Patient Management
Discharged Patient Management 20.1 Generating Patient Data NOTE • Earlier stored data will be overwritten by later ones if the equipment capacity is reached. 20.2 Accessing the Discharged Patient Mode WARNING • Patient therapy and monitoring automatically end when you access the Discharged Patient mode. The equipment automatically restarts and takes effects changes after the Discharged Patient mode. - Page 64 This page intentionally left blank. 20 - 2...
-
Page 65: Data Communication
Data Communication 21.1 Data Communication Safety Information CAUTION • Wireless network design, deployment, debugging, and maintenance should be executed by Mindray service personnel or authorized technicians. • Always deploy the wireless network according to local wireless regulations. • Using 5 GHz frequency band is recommended whenever possible. There are more interference sources in 2.4 GHz frequency band. - Page 66 This page intentionally left blank. 21 - 2...
-
Page 67: Configuration Management
Configuration Management 22.1 Configuration Management Introduction WARNING • Accessing the Configuration mode is password protected. Patient therapy and monitoring automatically end when you access the Configuration mode. • The configurations must be changed by authorized personnel only. • Never connect the equipment with the patient when accessing the configuration management. 22.2 Changing Configurations NOTE... - Page 68 This page intentionally left blank. 22 - 2...
-
Page 69: Battery
Battery 23.1 Battery Safety Information WARNING • Keep batteries out of children’s reach. • Use only specified battery. Use of a different battery may present a risk of fire or explosion. • Keep the batteries in their original package until you are ready to use them. •... -
Page 70: Checking Battery Performance
23.4 Checking Battery Performance NOTE • Life expectancy of a battery depends on how frequent and how long it is used. When properly used, the lithium-ion battery has a useful life of approximately two years. If improperly used, its life expectancy can be shorten. -
Page 71: Care And Cleaning
• Do not mix disinfecting solutions, as hazardous gases may result. • Mindray is not liable for the efficacy of the specified cleaners, disinfectants, or methods as a means for controlling infection. Refer to your hospital for infection controlling. •... -
Page 72: Cleaning And Disinfecting The Accessories
Never clean or disinfect the connectors and metal parts. • Use only Mindray approved cleaners and disinfectants and methods listed in this section to clean or disinfect the accessories. Warranty does not cover damage caused by unapproved substances or methods. -
Page 73: Maintenance
At the end of its service life, the equipment, as well as its accessories, must be disposed of in compliance with the guidelines regulating the disposal of such products. If you have any questions concerning disposal of the equipment, please contact Mindray. NOTE •... -
Page 74: User Test
test load, the prompt “Test load not connected with cable” is displayed when the auto test passes. This means that the equipment only passes the internal discharge test, but not pass the external discharge test connected with the test load. 25.2.2 User Test WARNING... -
Page 75: A Specifications
Specifications Safety Specifications A.1.1 Safety Classifications The equipment is classified, according to IEC 60601-1: Type of protection against electrical shock Class I Degree of protection against electrical Type BF defibrillation proof for CO monitoring and external defibrillation. shock Type CF defibrillation proof for ECG, SpO , NIBP and internal defibrillation. -
Page 76: Power Supply Specifications
Complies with requirements for medical devices of 6.3.4.2, EN1789 (10.1.3, IEC60601-1-12): Peak acceleration: 1000m/s (102g) Duration: 6ms Pulse shape: half-sine Number of shocks: 3 shocks per direction per axis (18 shocks in total) Bump Complies with the requirements for road ambulances of 6.3.4.2, EN1789. Peak acceleration: 15g Pulse duration: 6ms Number of bumps: 1000... -
Page 77: Charger Station Specifications
Battery charge time Charged by the equipment • Less than 3 hours to 90% and less than 4 hours to connected to the external power 100% with equipment turned off. supply • Less than 5 hours to 90% and less than 6 hours to 100% with equipment turned on. -
Page 78: Physical Specifications
Physical Specifications Dimensions (W × D × H) 285 mm×170 mm×265 mm (including the handle, excluding external paddles) Maximum weight 4.2 kg (the equipment is configured with 3-/5-lead ECG, manual defibrillation and one battery) Hardware Specifications A.4.1 Display Screen Screen type Capacitive, multi-point color touchscreen Screen size 8 inch... -
Page 79: External Connectors
A.4.5 External Connectors Power input 1, AC power input with equipotential grounding terminal, connects the external power supply. Multifunctional connector 1, connects a CPR sensor, or a cable for analog output or synchronized cardioversion. USB connector 1, USB 2.0, connects USB devices. Network connector 1 RJ45 connector, 100 Base-TX, IEEE 802.3, connects a standard network cable. -
Page 80: Communication Specifications
Communication Specifications A.6.1 Wi-Fi Specifications (Wlink as Station) Protocol IEEE 802.11a/b/g/n Modulation mode BPSK, QPSK, 16QAM, 64QAM Operating frequency 2412 MHz to 2472 MHz 5180 MHz to 5320 MHz, 5500 MHz to 5700 MHz, 5745 MHz to 5825 MHz Wireless baud rate IEEE 802.11a: 6 Mbps to 54 Mbps IEEE 802.11b: 1 Mbps to 11 Mbps IEEE 802.11g: 6 Mbps to 54 Mbps... -
Page 81: Cellular Specifications
Test conditions Meets the following conditions simultaneously: • Number of the equipments supported by a single AP: ≤ 3 • Each equipment can communicate with the CMS. • The weakest strength of the AP signal where the equipment is located is not less than -65 dBm. -
Page 82: Bluetooth Specifications
A.6.3 Bluetooth Specifications Protocol Bluetooth low energy 5.0 Modulation mode GFSK Operating frequency 2402 MHz to 2480 MHz Channel spacing 2 MHz Wireless baud rate 2 Mbps, 1 Mbps, 125kbps Output power ≤20 dBm Data security AES128 Distinct vision distance The distinct vision distance between the equipment and the Pad: ≥... - Page 83 Shocks: 1, 2, 3, configurable; Meeting AHA/ERC guidelines 2015 by default AED ECG analysis performance Refer to C Mindray Shockable Rhythm Analysis Algorithm. 360 J defibrillation waveform into impedance of 25Ω, 50Ω, 75Ω, 100Ω, 125Ω, 150Ω, 175Ω Time (ms) A - 9...
- Page 84 Impedance Selected energy Accuracy 25Ω 50Ω 75Ω 100Ω 125Ω 150Ω 175Ω ±10% or ±2J, whichever is greater 10 J 15 J 20 J 25 J 30 J 50 J 70 J 100 J 120 J 150 J 170 J 200 J 300 J 360 J Manual Defib...
-
Page 85: Cpr Compression Specifications
NOTE • The equipment startup time in the fast startup mode is less than 2s. A.7.2 CPR Compression Specifications Compressions from CPR Sensor Compression rate Measurement range: 40 to 160 cpm Accuracy: ±2 cpm Compressions from Electrode Pads Compression rate Measurement range: 60 to 200 cpm Accuracy: ±3 cpm A.7.3... - Page 86 6.25mm/s, 12.5 mm/s, 25 mm/s, 50 mm/s, less than ± 5% error Sweep speed Bandwidth (-3dB) Diagnostic mode: 0.05 to 150 Hz Monitor mode: 0.5 to 40 Hz Therapy mode: 1 to 20 Hz ST mode: 0.05 to 40 Hz High Freq Cut-off (for 12-lead 350 Hz, 150Hz, 35Hz, 20Hz, selectable ECG analysis):...
- Page 87 Resolution 1 bpm Sensitivity 200 μV (lead II) Heart rate averaging In compliance with the requirements in Clause 201.7.9.2.9.101 b) 3) of IEC 60601-2-27, the following method is used: If the last 3 consecutive RR intervals are greater than 1200 ms, the 4 most recent RR intervals are averaged to compute the HR.
-
Page 88: Ecg Specifications (From Therapy Accessories)
Measurements Heart rate, PR interval, QRS duration, QT/QTc interval, P/QRS/T axis and diagnosis statement A.8.2 ECG Specifications (from Therapy Accessories) Patient connection Paddles or multifunction electrode pads ECG inputs Paddles or Pads Display sensitivity 1.25 mm/mV (×0.125), 2.5 mm/mV (×0.25), 5 mm/mV (×0.5), 10 mm/mV (×1), 20 mm/mV (×2), 40mm/mV (×4), Auto, less than ±... -
Page 89: Resp Specifications
Heart rate averaging In compliance with the requirements in Clause 201.7.9.2.9.101 b) 3) of IEC 60601-2-27, the following method is used: If the last 3 consecutive RR intervals are greater than 1200 ms, the 4 most recent RR intervals are averaged to compute the HR. Otherwise, heart rate is computed by subtracting the maximum and minimum ones from the most recent 12 RR intervals and then averaging them. -
Page 90: Spo 2 Specifications
A.8.4 Specifications Mindray SpO Module Standard Meet standards of ISO 80601-2-61 Measurement range 0 to 100% Resolution Response time < 30 s (normal perfusion, no disturbance, SpO value sudden changes from 70% to 100%) Accuracy* 70% to 100%: ±2%ABS (adult, pediatric) 70% to 100%: ±3%ABS (neonate) -
Page 91: Pr Specifications
±1%, to compensate for the theoretical effect on oximeter measurements of fetal hemoglobin in neonatal blood. A.8.5 PR Specifications PR from Mindray SpO Module Measurement range 20 to 300 bpm... -
Page 92: Nibp Specifications
Accuracy ±3 bpm (measured without motion) ±5 bpm (measured with motion) Refreshing rate ≤1 s PR from Nellcor SpO Module Measurement range 20 to 300 bpm Resolution 1 bpm Response time ≤30 s (normal perfusion, no disturbance, PR value sudden change from 25 to 250 bpm) Accuracy 20 to 250 bpm: ±3 bpm... -
Page 93: Co Specifications
Measurement range 30 to300 bpm Resolution 1 bpm Accuracy ±3bpm or ±3%, whichever is greater A.8.7 Specifications Standard Meet the standard of ISO 80601-2-55 Measurement range 0 to 150 mmHg Accuracy Full accuracy mode: 0 to 40 mmHg: ±2 mmHg 41 to 76 mmHg: ±5% of reading 77 to 99 mmHg:... - Page 94 This page intentionally left blank. A - 20...
-
Page 95: B Emc And Radio Regulatory Compliance
EMC and Radio Regulatory Compliance The equipment meets the requirements of IEC 60601-1-2: 2014. WARNING • Use of accessories, transducers and cables other than those specified or provided by the manufacturer of this device could result in increased electromagnetic emissions or decreased electromagnetic immunity of this device and result in improper operation. - Page 96 If the device is operated within the electromagnetic environment listed in Table Guidance and Declaration — Electromagnetic Immunity, the equipment will remain safe and provide the following essential performance: HR accuracy, Resp accuracy, SpO accuracy, PR accuracy, NIBP accuracy, CO accuracy, Pacing rate accuracy, Pacing output accuracy, energy accuracy, CPR function, alarm, data stored, user’s interface function.
- Page 97 Guidance and Declaration - Electromagnetic Immunity The equipment is suitable for use in the electromagnetic environment specified below. The customer or the user of the equipment should assure that it is used in such an environment. Immunity test IEC 60601 Test level Compliance level Electromagnetic environment - guidance...
-
Page 98: Radio Regulatory Compliance
Recommended Separation Distances between Portable and Mobile RF, Communications Equipment and This Equipment The equipment is intended for use in an electromagnetic environment in which radiated RF disturbance are controlled. The customer or the user of the equipment can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the equipment as recommended below, according to the maximum output power of the communication equipment. - Page 100 P/N: 046-024584-00(1.0)














Need help?
Do you have a question about the BeneHeart D30 and is the answer not in the manual?
Questions and answers