Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Richmar HIVADOT
- Page 1 DEEP OSCILLATION THERAPY DEVICE INSTRUCTION MANUAL...
- Page 2 HIVADOT® Deep Oscillation Therapy Device This user manual is published by Richmar, a division of Compass Health Brands. Richmar, a division of Compass Health Brands, does not guarantee its contents and reserves the right to improve d it at any time without prior notice.
-
Page 3: Table Of Contents
TABLE OF CONTENTS Legal Notice ____________________________________________ 4 Limited Warranty ________________________________________ 5 Foreword _______________________________________________ 7 Operator Qualifications ___________________________________ 7 Symbols _______________________________________________ 8 Precautionary Definitions __________________________________ 9 Warnings and Cautions____________________________________ 10 Product Descriptions _____________________________________ 14 Contraindications ________________________________________ 14 Technical Specifications ___________________________________ 15 Device Inspection ________________________________________ 16 Overview of Device and Accessories _________________________ 17 Operating Instructions ____________________________________ 21... -
Page 4: Legal Notice
(or excepts thereof) are prohibited without the prior consent of Richmar, a division of Compass Health Brands. Richmar, a division of Compass Health Brands reserves the right to change the software and associated data without notice. All other rights reserved. -
Page 5: Limited Warranty
Richmar will repair or replace the respective Product without charge. Richmar's sole obligation in the case of any breach of its warranty set forth in the manual shall be, at Richmar's option, to replace the Product with a new or factory certified refurbished product, without charge to Richmar's purchaser or to refund the purchase price. - Page 6 A claim must be made within the warranty period directly to Richmar or the company from whom you purchased the device. 2. An RMA number must be obtained from Richmar in order to receive replacements parts and/or return defective product under the warranty.
-
Page 7: Foreword
OPERATOR QUALIFICATIONS The HIVADOT® is intended for use by medical specialists and is only allowed to be used by an end user if a qualified and instructed medical clinician provides guidance and instructions. Specialists must have the basic physical and cognitive prerequisites such as vision, hearing, and literacy. -
Page 8: Symbols
SYMBOLS Symbols on the medical device Manufacturer Correct Disposal of This Product (Waste Electrical & Electronic Equipment) Statement: Contact the local authorities to determine the proper method of disposal of potentially bio-hazardous parts Medical Device and accessories. Medical Device Type BF applied part complying with IEC 60601-1 This symbol indicates that this device is a Class II equipment accord- ing to IEC 60601-1 (when charging) Refer to instruction manual/ booklet... -
Page 9: Precautionary Definitions
PRECAUTIONARY DEFINITIONS The precautionary instructions found in this section and throughout this manual are indicated by specific symbols. Understand these symbols and their definitions before operating this equipment. The definition of these symbols is as follows: CAUTION Text with a “CAUTION” indicator will explain possible safety infractions that could have the potential to cause minor to moderate injury or damage to equipment. -
Page 10: Warnings And Cautions
WARNINGS AND CAUTIONS Please read the user manual entirely before using the HIVADOT® and take care of what follows: WARNING • Read, understand, and practice the precautionary operating instructions. Know the limitations and hazards associated with using the HIVADOT®. Observe the precautionary and operational decals placed on the unit. - Page 11 • DO NOT use this unit for purposes other than treatment indicated in this manual. • DO NOT use the HIVADOT® with high frequency surgical equipment on the patient. It will cause unstable output when the unit is close to the high frequency equipment (in the same room and without shield).
- Page 12 • If the unit is not functioning properly or you feel discomfort, immediately stop using the unit. If you feel any discomfort with your body or skin, consult the doctor and follow his/her instructions. • The adhesive electrodes and membranes are for single patient use only, DO NOT use on another patient to prevent infection.
- Page 13 CAUTION • ALWAYS check the device and the accessories for damage before use. • Take care not to allow water to enter the device. Liquid penetration Medical Device could damage the device. • DO NOT use volatile liquids, such as paint thinner or benzene, because they may damage the plastic case.
-
Page 14: Product Descriptions
PRODUCT DESCRIPTION The HIVADOT® is a pulsed electric therapeutic electrostatic field which is built up within the applicator and the patient’s tissue. Due to the movement of the hand applicator or the therapists’ gloved hands, a vibrating or pumping effect with deep impact is induced in the patient’s tissue. -
Page 15: Technical Specifications
TECHNICAL SPECIFICATIONS GENERAL Product Name HIVADOT® Product Model DO1009 Device Dimensions (W×L×H) 3.01" x 5.65" x 1.4" (76.5 x 143.5 x 35.5mm) Device Weight 0.5 lbs Interface 2.8" LCD screen Software Release Version PERFORMANCE Channel: Output Waveform Symmetrical biphasic pulse... -
Page 16: Device Inspection
5.5cm Oscillating Head (DO1099APP5) each 10cm Oscillating Head (DO1009APP10) each MultiStim Electrodes pack (400-8772) Box of Vinyl Gloves (VGPF3003) Instruction Manual each Zipper Carrying Case (DO1009BAG) each HIVADOT® replacement accessories are available for purchase. Please contact your distributor for more details. -
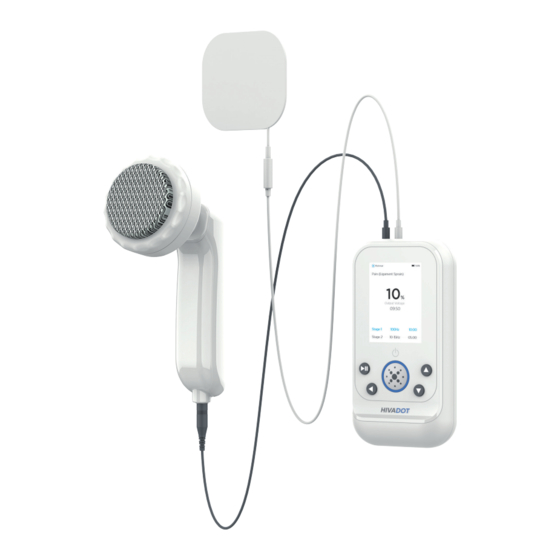
Page 17: Overview Of Device And Accessories
OVERVIEW OF DEVICE AND ACCESSORIES Output port 1* 6. UP button 2. Output port 2* DOWN button 3. Type C power adapter port 8. START/PAUSE/SKIP 4. Display screen Phase button 5. Power Button and Select 9. Back/STOP button Button *NOTE: Both output ports on the top of the device can accommodate either the white or grey cord, interchangeably. - Page 18 LEAD WIRES Pin Connection Lead wires for Electrodes (White - 2.0mm plug) - 2/pk Item #: DO1009PINLW Applicator/Titanium Element Lead Wire (Grey - 4.0mm plug) - 2/pk Item #: DO1009APPLW...
- Page 19 OSCILLATING HEADS 10cm Oscillating Head 5.5cm Oscillating Head Detachable oscillating heads 2. Applicator Handle 3. Applicator Connection 4. Applicator/Titanium Element Lead Wire Connector ADHESIVE ELECTRODE...
- Page 20 TITANIUM NEUTRAL ELEMENT VINYL GLOVES (100/bx) HIVADOT® STAND NOTE: Use replacement parts only supplied by the manufacturer. Contact your local dealer to place an order.
-
Page 21: Operating Instructions
OPERATING INSTRUCTIONS 1. PREPARING BEFORE THERAPY Charging the Device The HIVADOT® device will come with a partial charge. In order to complete a treatment, it is recommended to charge the device once it decreases to lower than a 30% charge. - Page 22 Treatment Methods WARNING DO NOT turn the device ON until you understand and are familiar with each Treatment Method option indicated below by referencing Correct Cable Connection section in the manual. Medical Device There are several ways to apply treatment: •...
- Page 23 Option #B Clinician Set up: Clinician can choose to use applicator attachment, instead of gloves, for hard to reach or uncomfortable areas. Patient Set up: Holds the titanium element in one hand or has one electrode applied to their body WARNING •...
-
Page 24: Start Treatment
CORRECT CABLE CONNECTION Depending on the treatment method chosen on the previous pages (Clinician Assisted Options or Self Treatment Options), the connection of lead wires to the device, and then to the accessories, is important for the device to emit the electrostatic pulses. IMPORTANT: If the method chosen requires connection to an oscillating head applicator or the titanium element, it must connect to a GREY... -
Page 25: Treatment Start Up
*Each phase is only 1 minute (total of 3 mins for complete demo) 3. TREATMENT START UP There are two options to choose from: • Custom Protocols • Preset Protocols The HIVADOT® allows clinicians to choose and save their own custom parameters to use in the future. - Page 26 Custom Protocol Setup STEP 1: Select a Favorite From the main menu, Custom Protocols should be highlighted blue. Press the [ ] to advance to the next screen. 2. Select one from Favorites 1-5 by pressing the up [ ▲ ] or down [ ▼...
-
Page 27: Parameter Changes
PARAMETER CHANGES Adjust Output Frequency (Hz)*: Press [ ] to highlight Hz parameter, blue, instead of blue bar across an entire stage. 2. Press [ ] again and the blue highlight will start blinking. This indicates it is ready to change the parameter. 3. -
Page 28: Treatment Screen Instructions
TREATMENT SCREEN INSTRUCTIONS Preset Protocol Setup The HIVADOT® has 31 programmed protocols to choose from (not including Demo). 1. From the Home Screen, press [ ▼ ] until the desired market is highlighted blue. There are 4 options to choose from: •... - Page 29 Treatment Screen Once in the treatment screen, press the [ ▲ ] to increase the intensity (output voltage) desired. Press the [ ] to start the treatment. Then the timer will start counting down. After the first stage is complete, the device will emit a beeping sound to let the patient know, it is advancing to the next stage.
- Page 30 Pausing Treatment Press the [ ] button and the device will stop the treatment time from counting down so therapy can continue from the time it was paused and not start the total treatment time over. Press the button again to restart treatment when ready. Stopping Treatment The device will either emit a beeping sound when all three phases are complete and treatment is done.
-
Page 31: Care And Maintenance
CARE AND MAINTENANCE Cleaning Make sure to turn off the device and remove all accessories (including the wall adapter and plug) from the device before cleaning and disinfecting. Clean the outer surface of the main device (making sure not to get any liquids in any of the attachment openings), the hand applicator, oscillating heads, the wall adapter and connection cables regularly with a clean, soft damp cloth or mild disinfectant wipe with 70% or less... -
Page 32: Routine Maintenance
ROUTINE MAINTENANCE Read this maintenance instruction thoroughly before doing any maintenance. Repairs and calibrations can only be performed by authorized service personnel approved by the manufacturer. WARNING To preserve the product warranty, functionality of device and product safety, it is recommended to only use replacement parts provided by the manufacturer. -
Page 33: Battery Replacement
BATTERY REPLACEMENT Rechargeable batteries have a limited number of charge cycles and may eventually need to be replaced. Please replace the battery if its power depletes rapidly or needs longer than usual to be recharged.* * A completely depleted battery should take no longer 2-5 hours for a full charge. -
Page 34: Disposal
DISPOSAL For environmental reasons, DO NOT dispose of the device in the household waste at the end of its useful life. Dispose of the device at a suitable local collection or recycling point. Dispose of the device in accordance with local state regulations. If you have any questions, please contact the local authorities responsible for waste disposal. -
Page 35: Troubleshooting
TROUBLESHOOTING NOTE: If the following measures fail to alleviate the problem, please call Tech Support at 888-549-4945, Option 2 Problem Possible Cause Solution No response after The battery is depleted. Charge the device (see section, pressing and "Preparing Before Therapy"). holding the ON/ OFF button. -
Page 36: Emc Guidance
• Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the HIVADOT®, including cables specified by the manufacturer. Otherwise, degradation of the performance of this equipment could result. -
Page 37: Cable Information
Guidance and manufacturer’s declaration - electromagnetic emissions The HIVADOT® is intended for use in the electromagnetic environment specified below. The customer of the user of the HIVADOT® should assure that it is used in such an environment. Emissions Test... - Page 38 Guidance and manufacturer’s declaration - electromagnetic immunity The HIVADOT® is intended for use in the electromagnetic environment specified below. The customer or the user of HIVADOT® should assure that it is used in such an environment. Immunity test IEC 60601 test level...
- Page 39 Guidance and manufacturer’s declaration - electromagnetic immunity (cont’d) The HIVADOT® is intended for use in the electromagnetic environment specified below. The customer or the user of HIVADOT® should assure that it is used in such an environment. Immunity test IEC 60601 test level...
- Page 40 Guidance and manufacturer’s declaration - electromagnetic immunity (cont’d) The HIVADOT® is intended for use in the electromagnetic environment specified below. The customer or the user of HIVADOT® should assure that it is used in such an environment. Immunity test IEC 60601 test...
- Page 41 Table: Test specifications for ENCLOSURE PORT IMMUNITY to RF wireless communications equipment Test Band Service Modulation Maximum Distance Immunity frequency (MHz) power (W) TEST (MHz) LEVEL (V/m) 380 to TETRA Pulse Modulation 18Hz 430 to GMRS 460, ± 5 Hz FRS 460 deviation 1 kHz sine...
- Page 42 Table: Test specifications for ENCLOSURE PORT IMMUNITY to RF wireless communications equipment Test Band Service Modulation Maximum Distance Immunity frequency (MHz) power (W) TEST (MHz) LEVEL (V/m) 5240 5100 to WLAN Pulse 5500 5800 802.11 Modulation 5785 217 Hz NOTE: a) For some services, only the uplink frequencies are included.
-
Page 43: Assistance
Any repairs must be performed by the manufacturer or by an authori- zed repair technician approved by the manufacturer. For assistance on ordering replacement parts or technical support, please contact: Richmar, a division of Compass Health Brands Ph: 888-549-4945 6753 Engle Road Middleburg Heights, OH 44130 www.richmarweb.com... - Page 48 Manufactured for: Made in China 3541.22.04.A ©2022 Compass Health Brands Corp.
















Need help?
Do you have a question about the HIVADOT and is the answer not in the manual?
Questions and answers