Table of Contents
Advertisement
CAUTION:
Read all warnings, cautions, and instructions provided with this equipment before using.
Read the instructions, warnings, and cautions provided with the Tag Applicator and LOCalizer Surgical
Probe before using. Specific instructions pertaining to Tag implantation and preparation and use of the
Surgical Probe are not included in this Manual.
DESCRIPTION
RFID Localization System
The Tag Applicator, LOCalizer™ Reader, and LOCalizer Surgical Probe are components of the RFID Localization
System. The Tag is intended to be placed in breast tissue, within 6 cm of the breast surface, using the Tag
Applicator. The Tags, when used in conjunction with the LOCalizer Reader and LOCalizer Surgical Probe, can
be used as a guide for the surgeon to follow in the excision of tissue.
RFID Localization System (RFLS) components are listed below:
System Component
Description
alizer
LOC
Reader
RFID Reader
alizer
LOC
Surgical Probe
Attachment probe for use with LOC
Tag Applicator
Needle applicator with preloaded RFID Tag
* XX indicates the length of the applicator needle in cm. Contact distributor for available sizes in your area.
The LOCalizer Instrument Drape (HB120) is provided separately for use with the LOCalizer Reader in a
sterile environment.
LOCalizer Reader
The LOCalizer Reader locates and reads (RFID) Tags which have been implanted using the Tag Applicator.
The LOCalizer Reader displays the distance between the Tag and the Probe under use.
Tag location is also indicated by an audible sound of which the pitch and volume increases in proportion to a decrease
in the "LOCalizer to Tag" distance.
The LOCalizer Reader is a portable, battery-operated system and is supplied non-sterile. The Tag Applicator and
LOCalizer Surgical Probe are provided sterile.
This manual includes guidelines for the use of the Probe in the sterile eld.
1
Part Number
HB100
alizer
RFID Reader
HB110
HB200-XX*
HB300-XX*
INDICATIONS FOR USE
The Tag of the RFLS is intended for percutaneous placement in the breast to mark (>30 days) a lesion intended for
surgical removal. Using image guidance (such as ultrasound or radiography) or aided by non-imaging guidance
(RFLS), the RFID Tag is located and surgically removed with the target tissue.
The RFLS is intended only for the non-imaging detection and localization of the Tag that has been implanted in a
lesion intended for surgical removal.
The RFID Localization System is not intended for use under conditions where breast lesion localization
is contraindicated.
The RFID Localization System is not intended for use in the heart, eyes, brain or spinal cord.
The Tag should not be placed in a tissue site with clinical evidence of infection.
The Tag should not be placed in muscle tissue.
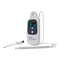
LOCALIZER READER OVERVIEW
The LOCalizer Reader (Figure 1) is composed of:
1 Handheld LOCalizer Reader
2 AA Alkaline batteries (IEC-LR6)
The LOCalizer Reader includes an integrated Loop Probe (A) that is used to locate and read Tags from the skin
surface. It can also be used with the LOCalizer Surgical Probe attachment (D) to locate and read Tags within the
surgical incision. Probe details are included in Table 1. Subsequent sections of this manual describe in detail probe
and LOCalizer Reader use.
Figure 1. LOCalizer Reader with Surgical Probe connected (Instrument Cover not shown)
A
Loop Probe (integrated)
B
Screen and Control Panel
C
Battery Cover
D
Surgical Probe
E
Surgical Probe Connector
2
Advertisement
Table of Contents

















Need help?
Do you have a question about the LOCalizer and is the answer not in the manual?
Questions and answers