Table of Contents
Advertisement
Artwork consists of:
Artwork master contains the following file(s):
AW-15026-002_010_02.zip
AW-15026-002_010_02.pdf
Artwork prints black and white.
REV AUTHORED BY
S VAILLANCOURT 03/21/19
REV DRAFTED BY
S VAILLANCOURT 03/21/19
PROPRIETARY: This document contains
proprietary data of Hologic, Inc. No
disclosure, reproduction or use of any
part thereof may be made except by
written permission from Hologic.
REV. RELEASE DATE:
15 MAY 2019
•
Nine 8.25-inch x 11-inch sheets attached.
File Name
DATE
DATE
TITLE
TEXT, MYOSURE XL MODIFIED FOR
FLUENT, TEXT, EN OUS,
Description
Source and supplier print file
View file
DOCUMENT NUMBER
AW-15026-002
SIZE A
REV
010
SHEET 1 OF 1
Form ENG-0034-T01, Rev. 005
Advertisement
Table of Contents

Summary of Contents for Hologic MyoSure XL
- Page 1 DATE S VAILLANCOURT 03/21/19 PROPRIETARY: This document contains TITLE DOCUMENT NUMBER proprietary data of Hologic, Inc. No TEXT, MYOSURE XL MODIFIED FOR disclosure, reproduction or use of any AW-15026-002 part thereof may be made except by FLUENT, TEXT, EN OUS, written permission from Hologic.
- Page 2 WARNING: Exercise extreme caution when resecting tissue in patients who have implants that extend into the uterine cavity. • Do not use the MyoSure XL Tissue Removal Device for Fluent to resect tissue that is adjacent to an implant. When resecting tissue in patients that have implants, assure that: •...
-
Page 3: Electromagnetic Safety
Electromagnetic Safety The MyoSure XL Tissue Removal Device for Fluent is only to be used with the Fluent Fluid Management System. The Fluent Fluid Management System needs special precautions regarding electromagnetic safety. -
Page 4: Electrical Connections
MyoSure XL Tissue Removal Device for Fluent ® Impact of Mobile and Portable HF Communication Devices The emission of high frequency energy by mobile communication devices may impact the function of the electrical medical device. Operating such devices (e.g., cell phones, GPS phones) in the proximity of the electrical medical device is prohibited. Electrical Connections Do not touch electrical connections identified with this warning label. - Page 5 MyoSure XL Tissue Removal Device for Fluent ® Immunity test IEC 60601 Compliance level Electromagnetic environment - guidance test level Voltage dips, short interruptions and 0% UT (100% dip in the 0% UT (100% dip in the Mains power quality should be that of a typical voltage variations on power supply UT) for ½...
-
Page 6: Recommended Separation Distances
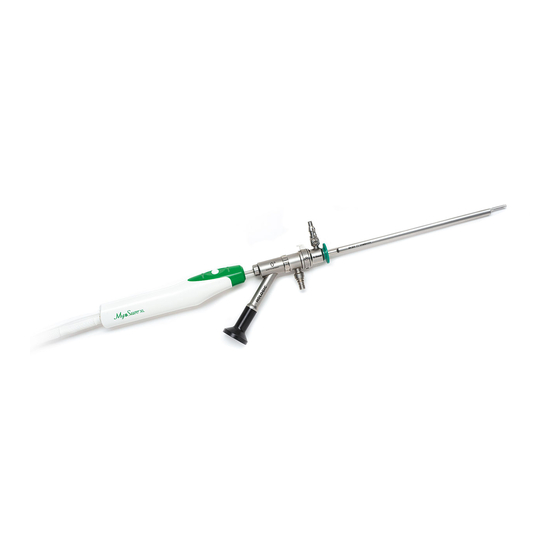
Tissue Removal Device for Fluent: 50-601XL/50-603XL The MyoSure XL Tissue Removal Device for Fluent is shown in Figure 1. It is a hand-held unit which is connected to the Fluent Fluid Management System via a 6-foot (1.8-meter) flexible drive cable and to the Out-FloPak via a 10-foot (3-meter) suction tube. -
Page 7: Operation
MyoSure XL Tissue Removal Device for Fluent ® Connecting the Tissue Removal Device to the Fluent Fluid Management System 1. Remove the tissue removal device (REF 50-601XL/50-603XL) from the sterile package. 2. Sterile person hands the flexible drive cable and suction tube to the non-sterile person. 3. -
Page 8: Troubleshooting
4. The foot pedal is connected to the front of the Fluent Fluid Management System. 5. The suction tubing is connected. If excess bending force is applied to the MyoSure XL Tissue Removal Device for Fluent, the system may temporarily stop to prevent further damage. - Page 9 Technical Support and Product Information Contact Hologic Technical Support if the MyoSure XL Tissue Removal Device for Fluent fails to operate as intended. If product is to be returned to Hologic for any reason, Technical Support will issue a Returned Materials Authorization (RMA) number and biohazard kit if applicable. Return the MyoSure XL Tissue Removal Device for Fluent according to the instructions provided by Technical Support.
- Page 10 Do not use if package is damaged Hologic, MyoSure, and associated logos are registered trademarks of Hologic, Inc. and/or its subsidiaries in the United States and other countries. All other trademarks, registered trademarks, and product names are the property of their respective owners.












Need help?
Do you have a question about the MyoSure XL and is the answer not in the manual?
Questions and answers