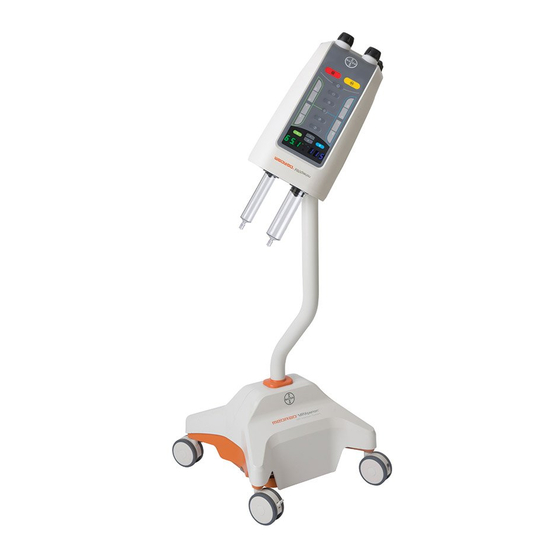
Bayer HealthCare MEDRAD MRXperion Operation Manual
Prefilled syringe adapter (pfa) mr injection system
Hide thumbs
Also See for MEDRAD MRXperion:
- Operation manual (126 pages) ,
- Operating manual (98 pages) ,
- Instructions for use manual (87 pages)
Table of Contents
Advertisement
Advertisement
Table of Contents

Subscribe to Our Youtube Channel
Summary of Contents for Bayer HealthCare MEDRAD MRXperion
- Page 1 Operation Manual Prefilled Syringe Adapter (PFA)
- Page 3 Operation Manual Report any serious incident that has occurred in relation to this device to Bayer (radiology.bayer.com/contact) and to your local European competent authority (or, where applicable, to the appropriate regulatory authority of the country in which the incident has occurred).
- Page 4 MEDRAD® MRXperion Prefilled Syringe Adapter...
-
Page 5: Table Of Contents
Operation Manual 1 Overview ......................1 - 1 1.1 Indications for Use ...................................1 - 1 1.2 Contraindications..................................1 - 1 1.3 Symbols.......................................1 - 1 1.4 Prefilled Syringe Adapter (PFA) Components ........................1 - 3 1.5 Prefilled Syringe Adapter Usage ............................1 - 4 1.6 Home Screen ....................................1 - 5 1.7 Injector Head Overview ................................1 - 6 1.8 PFA Installation Features ...............................1 - 7 2 Configuration....................2 - 9... - Page 6 MEDRAD® MRXperion Prefilled Syringe Adapter...
-
Page 7: Overview
Operation Manual 1 - 1 Overview For complete operational information, warnings, and cautions, please refer to the MEDRAD® MRXperion MR Injection System operation manual. This manual applies to the MEDRAD® MRXperion Prefilled Syringe Adapters (Catalog Numbers: MRXP PFA GN, MRXP PFA PE, MRXP PFA YW, MRXP PFA GY) for use with MEDRAD®... - Page 8 1 - 2 MEDRAD® MRXperion Prefilled Syringe Adapter Atmospheric pressure range (ISO 15223-1, 5.3.9) Date of manufacture (ISO 15223-1, 5.1.3) Serial number (ISO 15223-1, 5.1.7) Catalog number (ISO 15223-1, 5.1.6) This side up (ISO 7000, 0623) Keep dry (ISO 15223-1, 5.3.4) Fragile, handle with care (ISO 15223-1, 5.3.1) Part number Net weight (ISO 7000, 1321B)
-
Page 9: Prefilled Syringe Adapter (Pfa) Components
Operation Manual 1 - 3 Indicates that the information is a warning. Warnings advise you of circumstances that WARNING could result in injury or death to the patient or operator. Read and understand the warnings before operating the injection system. Indicates that the information is a caution. -
Page 10: Prefilled Syringe Adapter Usage
1 - 4 MEDRAD® MRXperion Prefilled Syringe Adapter 1.5 Prefilled Syringe Adapter Usage There are four color-coded prefilled syringe adapters available from Bayer: Supplier Name PFA # Volume (mL) Magnevist 5, 10 Bayer Gadovist 5, 7.5, 10 Primovist 5, 10 Eisai Prohance¹... -
Page 11: Home Screen
Operation Manual 1 - 5 1.6 Home Screen Name Description Launch Menu Select to access Setup, Calculators, VirtualCare (if installed), Help, and Shutdown. Fluids Select to access the Information screen and choose the Fluid A source type (contrast). When a PFA is installed, an adapter with the color and number of the installed PFA will display. -
Page 12: Injector Head Overview
1 - 6 MEDRAD® MRXperion Prefilled Syringe Adapter 1.7 Injector Head Overview Name Name Volume Indicator (Side A or B) Arm Button B Button Start/Hold Button A Button Abort Button Prime Button KVO Button I Checked for Air Confirmation Button Test Inject Button Enable Piston Control Button Manual Knobs... -
Page 13: Pfa Installation Features
Operation Manual 1 - 7 1.8 PFA Installation Features The system is designed with four features that decrease the time and steps to install and remove the PFA to the injector head. Non-rotational orientation: When installing a PFA onto the injector head, alignment is unnecessary. Push the PFA into the Side A opening. - Page 14 1 - 8 MEDRAD® MRXperion Prefilled Syringe Adapter...
-
Page 15: Configuration
Operation Manual 2 - 9 Configuration 2.1 Enabling Prefilled Syringe Adapter (PFA) Feedback System Setup enables the operator to configure settings that affect operation of the overall system. From the Launch Menu at the bottom left of the Home screen, select Setup, then select System Setup. On the second page of System Setup, select Prefilled Syringe Adapter (PFA) Feedback. - Page 16 2 - 10 MEDRAD® MRXperion Prefilled Syringe Adapter Edit information for each of the following items by selecting a tab (2) on the left of the screen and entering the parameters: Figure 2 - 1: Adding a New Contrast Type Contrast Name: Select an existing contrast name from the list (3) or add a new contrast name by selecting Add New (4) and entering the name of the contrast using the keyboard window that will appear on the display.
-
Page 17: Editing An Existing Contrast Type
Operation Manual 2 - 11 2.2.2 Editing an Existing Contrast Type Select Contrast Types on the Fluid Delivery Setup screen. Select a saved Contrast Type (1) on the Contrast Configuration screen and select Edit (2). Figure 2 - 2: Select an Existing Contrast Type to edit Edit information for each of the following items by selecting a tab on the left of the screen and entering the parameters (See Figure 2 - 3): Contrast Name: Select an existing contrast name from the list (3) or add a new contrast name by selecting Add... -
Page 18: Contrast Type Management
2 - 12 MEDRAD® MRXperion Prefilled Syringe Adapter Figure 2 - 3: Editing an Existing Contrast Type 2.2.3 Contrast Type Management When two or more contrast types have been saved, the order can be changed by using the Move Up and Move Down buttons on the Contrast Configuration screen. -
Page 19: Preparing For An Injection, Arming, And Injecting
Operation Manual 3 - 13 Preparing for an Injection, Arming, and Injecting For complete operational information, warnings, and cautions, please refer to the MEDRAD® MRXperion MR Injection System operation manual. WARNING Biological Contamination Hazard - Serious patient and/or worker injury or death may result. Properly discard contrast and saline containers and disposable items after use (refer to disposable label for specifics), or if there is any possibility that contamination may have occurred. -
Page 20: Control Room Preparation
3 - 14 MEDRAD® MRXperion Prefilled Syringe Adapter 3.1 Control Room Preparation Select Fluids from the informatics panel on the left side of the Home screen. The Information screen will display. Figure 3 - 1: Select Fluids on the Home Screen On the Fluid A tab (1) on the Information screen, select the Source Type field (2). - Page 21 Operation Manual 3 - 15 The Home screen will now show the compatible PFA to use with the selected contrast type (if PFA Feedback is enabled). Figure 3 - 3: Compatible PFA Recommendation on Home Screen When a PFA is installed on the injector, the PFA Status Indicator (1) on the Home screen will show the PFA installed on Side A.
-
Page 22: Injection Preparation
3 - 16 MEDRAD® MRXperion Prefilled Syringe Adapter 3.2 Injection Preparation Set a protocol from the display. Confirm the protocol, then select Lock on the display. NOTE: Lock on the display changes to Arm and the injector head Protocol Lock indicator illuminates. Install the saline (Side B) syringe into the injector head. -
Page 23: Attach And Prime The Tubing
Operation Manual 3 - 17 Advance the adapter piston to the PFS plunger by pressing the Enable Piston Control button and using the Side A Low Speed Forward Piston Control. The top of the PFS should be in contact with the PFA. Figure 3 - 7: PFS in Contact with PFA NOTE: Load rate will automatically adjust when a PFA is installed. -
Page 24: Removing Disposables
3 - 18 MEDRAD® MRXperion Prefilled Syringe Adapter 3.4 Removing Disposables Retract the adapter piston: Press the A button on the injector head Select New Patient and acknowledge the pop-up on the display. Remove and discard disposables. NOTE: Do not discard the prefilled syringe adapter after use. -
Page 25: Inspection, Cleaning, And Disinfection Of The Pfa
Operation Manual 4 - 19 Inspection, Cleaning, and Disinfection of the PFA NOTICE: Electro-Mechanical Hazard - Equipment Damage may result. Do not soak or immerse the adapter in water. Immersing the adapter in water could damage the adapter. On the PFA, do not use cleaning agents containing quaternary ammonium compounds (e.g. dimethyl ethylbenzyl ammonium chloride), such as Sani-Cloth®... -
Page 26: Disinfecting The Pfa
4 - 20 MEDRAD® MRXperion Prefilled Syringe Adapter 4.3 Disinfecting the PFA Frequency: As needed or when visibly contaminated Material: Disinfection agent: Use Clorox Healthcare® Hydrogen Peroxide Cleaner Disinfectant Wipes (EPA registration #67619-25) If Clorox Healthcare Hydrogen Peroxide Cleaner Disinfectant Wipes are not available within your country, then use a disinfectant with 1.4% - 3% hydrogen peroxide Ensure the PFA has been thoroughly cleaned. -
Page 27: Troubleshooting
Operation Manual 5 - 21 Troubleshooting 5.1 Selected Source Type Not Compatible with PFAs from Bayer If the selected prefilled syringe is not compatible with PFAs, a message will appear on the screen that says “Prefilled Syringe not compatible with PFAs.” For a list of compatible prefilled syringes, refer to "Figure 1 - 1: on page 1-4". - Page 28 5 - 22 MEDRAD® MRXperion Prefilled Syringe Adapter...
-
Page 29: Specifications
Operation Manual 6 - 23 Specifications 6.1 Injector (Scan Room Unit) Dimensions with PFA Installed NOTE: Listed dimensions are approximate. 6.2 Prefilled Syringe Adapter (PFA) Dimensions NOTE: Listed dimensions are approximate. 11.7 in (30 cm) 0.5 lbs 2.2 in (0.2 kg) (6 cm) -
Page 30: System Capabilities With Pfa Installed
6 - 24 MEDRAD® MRXperion Prefilled Syringe Adapter 6.3 System Capabilities with PFA Installed VOLUME 0.5 mL to max. syringe volume in: PFA (Side A) (Programmable): 0.1 mL increments up to 20mL FLOW RATE 0.01 mL/s increments between 0.01 and 3.1 mL/s 0.01 to 10 mL/s in: (Programmable): 0.1 mL/s increments between 3.1 and 10mL/s... - Page 32 All patient data that appear in this document are fictitious. No actual patient information is shown. Bayer, the Bayer Cross, MEDRAD, MRXperion, and MEDRAD MRXperion are trademarks owned by and/or registered to Bayer in the U.S. and/or other countries. Other trademarks and company names mentioned herein are properties of their respective owners and are used herein solely for informational purposes.













Need help?
Do you have a question about the MEDRAD MRXperion and is the answer not in the manual?
Questions and answers