Table of Contents
Advertisement
Advertisement
Table of Contents

Subscribe to Our Youtube Channel
Summary of Contents for Zimmer Micro 5
- Page 1 Instructions for use Micro 5...
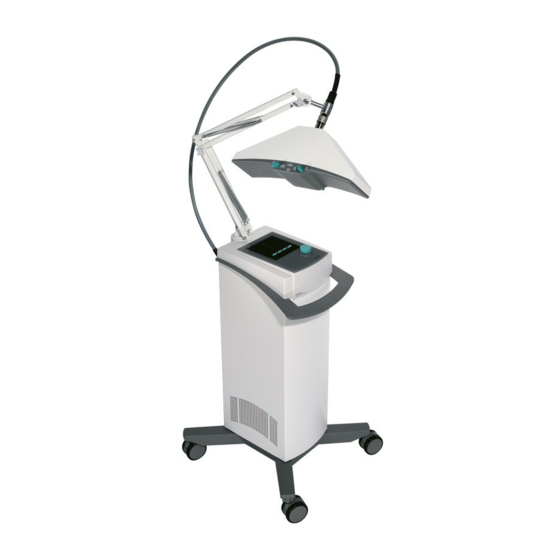
- Page 2 Figure Front of the device Fig. overall view Radiator head cable Radiator head connection Adjustable radiator head Adjustment knob for radiation field Housing with vents Cruciform base with device rollers...
- Page 3 Figure Rear of the device Fig. front plate Power display Frequency display Treatment clock Operating display Mains connection display Display, unpulsed Display, pulsed Start/stop button Operating mode button (unpulsed/pulsed) Time setting Intensity controller Test lamp...
- Page 4 Figure Screens and display Fig. rear Power inlet with mains connection switch and fuses Connection for radiator head cable Housing with vents...
- Page 5 Explanation of symbols In the instructions for use this symbol indicates “Danger”. In the instructions for use this symbol indicates “Caution” with regard to possible Caution damage of the device. Applied part B This symbol points out hazardous areas on the device. This symbol warns of high voltage.
-
Page 6: Table Of Contents
Explanation of symbols Page Indications / Contraindications Fehler! Textmar ke nicht definiert 1.1.1. Side effects 1.1.2. Application information 1.1.1. Warnings 1.1.1. Micro 5 - in brief 1.1.2. Device set-up 1.1.1. Default settings 1.1.2. Operation instructions Technical information Cleaning / Disinfection 1.1.3. - Page 7 EMC declaration 1.1.8. Valid for the device Micro 5. These instructions for use are an integral part of the device. They must be stored with the device and kept accessible at all times for anyone authorised to operate this device.
-
Page 8: Indications / Contraindications
Indications / Contraindications Indications Pain relief during Carpal Tunnel Syndrome Knee Osteoarthritis Shoulder pain Further treatment recommendations Pain relief at Chronic back pain and neck pain Damage to the shoulder joint Contraindications Acute and subacute thrombophlebitis ... -
Page 9: Side Effects
Side effects Side effects of No side effects are known if used correctly. microwave treatment Page 3... -
Page 10: Application Information
(electrical installation according to DIN VDE 0100 Part 710). The device must only be operated with the supplied power cable. The power cable must be protected against mechanical stress. Micro 5 is not suitable for use in areas with an explosive, flammable or combustive Caution atmosphere. - Page 11 the immediate vicinity or Micro 5 itself. The devices can be impeded in their action or suffer lasting damage. you leave a safety distance of 5 m from medical devices being operated at the same ...
-
Page 12: Micro 5 - In Brief
Micro 5 is easy to use, and its adjustable radiator head allows it to be used in various treatment situations. What are the other benefits of Micro 5? Two selectable pulse frequencies mean that the heat pulses can be clearly felt. The pulse method allows higher intensities to be applied at a constant average power level, enabling heat to penetrate deeper. -
Page 13: Device Set-Up
Then replace the clip. Connecting the device to a power Micro 5 may only be operated on a secure power supply for medical premises in supply application group 1 in accordance with VDE 0107. Only use the power cable supplied. -
Page 14: Default Settings
Micro 5 is ready for use. Direct the radiator head at the part of the body to be treated, or place it directly on the part. -
Page 15: Operation Instructions
Operation instructions Note: The following recommendations are only guidelines and must of course be adapted to the patient's individual situation. To switch on the device, place the mains connection switch (rear of the device) in position I. The device performs a self-test after it has been switched on. All display elements are controlled. - Page 16 After 5 seconds the operating display starts to glow steadily. Now the microwave output can be set and treatment can begin. Operating modes Micro 5 offers the following operating modes: unpulsed: max. 250 W pulsed 0.5 Hz low power: max.
- Page 17 Operation instructions Comply with the warnings in these instructions for use when positioning the radiator head! Adjusting the radiator head The radiation properties of the radiator head can be adjusted to the requirements of treatment. Adjust the knobs to produce the following radiation fields. Large field setting Circular field setting Half field left...
- Page 18 Patients with cardiac pacemakers must not undergo microwave treatment. The range of indications for which Micro 5 can be used is broader if the pulse operating mode option is selected. The low level of energy conversion means that there are no heat effects in this form of administration.
-
Page 19: Technical Information
Technical information Device type Microwave treatment device Operating frequency 2450MHz ± 25MHz High-frequency power at 60 unpulsed: max. 250W pulsed: max. 125W pulse-pause ratio 1:1 pulsed low power: max. 50W pulse-pause ratio 1:2 Accuracy ±20% The power figures relate to the average power output. The maximum power is 1200W. -
Page 20: Cleaning Disinfection
Cleaning Disinfection Before starting any maintenance and cleaning measures the device must always be switched off at the main switch and the mains cable must be disconnected. Make sure that during cleaning and disinfection no liquids penetrate the device. Do not use sprays. -
Page 21: Ce Mark
CE mark The device has a CE mark in accordance with the EC directive on medical devices 93/42/EEC. Manufacturer Zimmer MedizinSysteme GmbH Junkersstraße 9 89231 Neu-Ulm, Germany Tel. +49 731. 9761-0 Fax +49 731. 9761-118 www.zimmer.de Page 15... -
Page 22: Scope Of Delivery And Accessories
Scope of delivery / accessories Scope of delivery Item no.: 7100 Micro 5 basic device Item no.: 95.470.400 Adjustable radiator head, adjustable for three radiation fields (circular field, half field, large field) Item no.: 98.470.400 Radiator head cable Item no.: 93.471.200 Radiator head arm Item no.: 118... -
Page 23: Device Combinations
Device combinations For Micro 5 no combination devices are provided by the manufacturer. Anyone who combines devices against these guidelines and thus creates a medical system does so under his/her own responsibility. Page 17... -
Page 24: Safety And Maintenance
Fuses and other spare parts may be replaced only by trained service staff. Device service may only be performed by trained staff. All descriptions required for service can be seen in the Micro 5 service manual or can be requested from the manufacturer. Upon request, Zimmer MedizinSysteme will provide wiring diagrams, lists of components, descriptions, calibration instructions or other documents. -
Page 25: Functional Test
Functional test Functional test Once installation has been completed, check again whether the radiator head cable’s connections are properly inserted and secured by the clip. Then perform a functional test. Caution! Make sure that the plug connections are properly connected and that the clip is secure. -
Page 26: Safety Check / Metrological Check
Safety checks Metrological control In Germany, it is not necessary to perform either a safety check or a metrological control for the Micro5 device. In Germany, the Medical Device Operator Ordinance (MPBetreibV) as well as the Regulation of the German Employer's Liability Insurance Association on electrical systems and equipment (BGV A3), among others, apply in their respective current versions. -
Page 27: Fault Messages Troubleshooting Disposal
You may get in touch with them via your sales representative or via the main office in Neu-Ulm. Main office Zimmer MedizinSysteme GmbH Junkersstraße 9 89231 Neu-Ulm Tel. +49 731 / 9761-291 Fax +49 731 / 9761-299 export@zimmer.de... -
Page 28: Emc Declaration
Portable and mobile HF communication devices (such as mobile phones, cell phones) can affect medical electrical devices. Micro 5 may only be operated with the original power cable indicated in the list of the scope of delivery and accessories. Operation of the device with a different power cable can lead to increased emissions or reduced interference immunity of the device! Guidelines and manufacturer's declaration –... - Page 29 Guidelines and manufacturer's declaration – Electromagnetic immunity The Micro 5 device is intended to be operated in the electromagnetic environment as indicated below. The customer or user of the Micro 5 device should ensure that it is used in such an environment.
- Page 30 Guidelines and manufacturer's declaration – Electromagnetic immunity The Micro 5 device is intended to be operated in the electromagnetic environment as indicated below. The customer or user of the Micro 5 device should ensure that it is used in such an environment.
- Page 31 If the measured field strength in the location in which the Micro 5 device is used exceeds the above compliance levels, the Micro 5 device must be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the Micro 5 device.
- Page 32 Micro 5 Instructions for use Zimmer MedizinSysteme GmbH Junkersstraße 9 89231 Neu-Ulm, Germany Tel. +49 731. 97 61-291 Fax +49 731. 97 61-299 export@zimmer.de www.zimmer.de...
















Need help?
Do you have a question about the Micro 5 and is the answer not in the manual?
Questions and answers