Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for ResMed Positive Airway Pressure Device VPAP IV
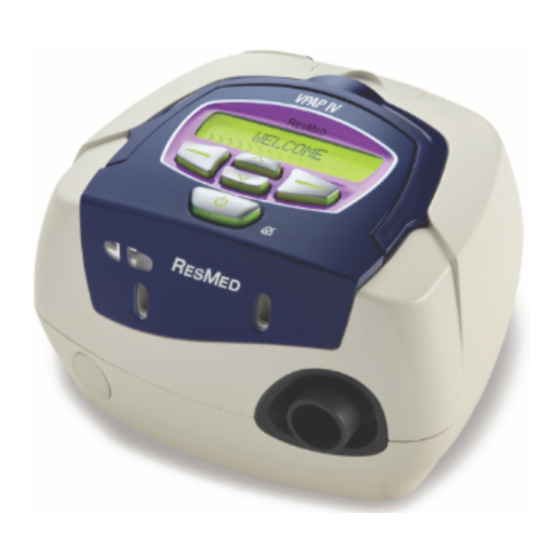
- Page 1 VPAP™ IV VPAP™ IV ST Positive AirwAy Pressure device User Guide English...
- Page 2 NZ 406925, NZ 406926, NZ 406927, NZ 406928, US D544598, US D557406, US D557407, US D560795, US D561891. Other designs pending. SmartStart, TiCONTROL, VPAP, and Vsync are trademarks of ResMed Ltd and SmartStart and VPAP are registered in U.S. Patent and Trademark Office.
-
Page 3: Table Of Contents
Contents ..........1 Introduction Contraindications Adverse Effects... -
Page 4: Introduction
2 m air tubing • Power cord • Travel bag • ResScan™ Data Card • ResMed Oxygen Connector Port. • Optional components include: 3 m air tubing • DC/DC Converter 24V/50W. • IV ST are intended to provide non-invasive ventilation... -
Page 5: Masks
Masks The following ResMed mask systems are recommended for use with these devices: Mask Type Nasal Masks Nasal Pillows Systems Full Face Masks For information on using masks, see your mask manual. For the latest available masks, see www.resmed.com. Humidifier If you are experiencing dryness of the nose, throat or mouth, the H4i heated humidifier is recommended for use with these VPAP devices. -
Page 6: Using The Vpap Iv And Vpap Iv St
Make sure the power cord and plug are in good condition and the • equipment is not damaged. Only ResMed air tubing and accessories should be used with the device. Do • not use electrically conductive or antistatic hoses. A different type of air tubing or accessory may alter the pressure the patient receives, reducing the effectiveness of the treatment. -
Page 7: Attaching A H4I Humidifier
Attaching a H4i Humidifier The H4i humidifier attaches to the front of a VPAP IV or VPAP IV ST device to provide heated humidification. These devices automatically detect the presence of the H4i and no other accessories are required for its use. For more information on using your H4i, please refer to the H4i user guide. -
Page 8: Using The Menus
Using the Menus The VPAP IV and VPAP IV ST provide a set of functions which are arranged in menus and submenus. Via the LCD screen, the menus and submenus allow you to view and change the settings for a particular function. To navigate and make selections: Press Press to enter a submenu and to apply an option choice. -
Page 9: How To Select The Mask Type
How to Select the Mask Type Scroll to MASK and select you require. The following table shows the setting that should be selected for each mask: Settings ULTRA MIR FULL ACTIVA SWIFT STANDARD MIRAGE SmartStart™ If your clinician has enabled SmartStart/Stop, your device will start automatically when you breathe into your mask and stop automatically when you take your mask off. -
Page 10: Stopping Treatment
After starting therapy, an introductory treatment screen will display: S mode S:RAMP 4.0-10.0 >>>> PS 6.0 example LK: 10L/min MV: 10.2 VT: 680 S**TiMn TiMx 2.0 Ti 1.5s SpO2: 98% HR : 60 Stopping Treatment To stop treatment at any time, remove your mask and press has enabled SmartStart/Stop, simply remove your mask and treatment will end. -
Page 11: Reminders On The Vpap Lcd
Reminders on the VPAP LCD Your clinician may have set your VPAP device to remind you about important events, such as when to replace your mask, when to insert your Data Card (if your device is Data Card enabled) and so on. The reminder message is displayed on the LCD and is visible if the device is not delivering therapy. -
Page 12: Using The Data Card
Using the Data Card If your clinician needs to review your treatment, they will ask you to use the Data Card to copy data from your VPAP device and return the card to them. Copying Data onto a Data Card Switch on your VPAP and wait until you see the standby (Ramp) screen. - Page 13 Note: You should not use your VPAP device while the aircraft is taking off or landing. Use with DC Power You must use a ResMed DC/DC Converter 24V/50W to connect your VPAP to a 12V or 24V DC power source. Contact your equipment supplier or ResMed for details.
-
Page 14: Cleaning And Maintenance
Cleaning and Maintenance You should regularly carry out the cleaning and maintenance described in this section. Refer to your mask and humidifier manuals for detailed instructions regarding the care of those devices. Daily Disconnect the air tubing and hang it in a clean, dry place until next use. Weekly Remove the air tubing from the VPAP device and the mask. -
Page 15: Servicing
ResMed. To ensure that your device continues to provide optimum performance it is recommended that this product be inspected by an authorised ResMed Service Centre five years from the date of purchase. Applicable ResMed warranty details are provided with the device at the time of original supply. -
Page 16: Troubleshooting
Troubleshooting If there is a problem, try the following suggestions. If the problem cannot be solved, contact your equipment supplier or ResMed. Do not attempt to open the device. Problem/Possible Cause No display Power is not connected. Insufficient air delivered from the VPAP device Ramp time is in use. - Page 17 Data Card: Settings Success Remove Card The settings were not updated. Solution Record error number and contact your ResMed service centre. Check that your air tubing is connected properly. Adjust headgear. Ensure that the Data Card is inserted with the arrow facing up as far as it can go.
-
Page 18: Technical Specifications
Technical Specifications Operating pressure range Maximum single fault steady state pressure Pressure measurement tolerance Flow measurement tolerance S, ST and T modes CPAP mode DECLARED DUAL-NUMBER NOISE EMISSION VALUES in accordance with ISO 4871: Sound pressure level Sound power level Dimensions (L x W x H) Weight Power supply... -
Page 19: General Warnings And Cautions
These VPAP devices should only be used with masks (and connectors by ResMed, or by a physician or respiratory therapist. A mask should not be used unless the VPAP device is turned on and operating properly. The vent hole or holes associated with the mask should never be blocked. - Page 20 Guidance and Manufacturer’s Declaration – Electromagnetic Emissions and Immunity Guidance and manufacturer’s declaration – electromagnetic emissions The VPAP IV and VPAP IV ST devices are intended for use in the electromagnetic environment specified below. The customer or the user of the VPAP device should assure that the device is used in such an environment. Emissions test RF emissions CISPR11 RF emissions CISPR 11 with serial adapter...
- Page 21 Guidance and manufacturer’s declaration – electromagnetic immunity The VPAP device is intended for use in the electromagnetic environment specified below. The customer or the user of the VPAP device should assure that the device is used in such an environment. IEC60601-1-2 test Immunity test level...
-
Page 22: Limited Warranty
ResMed shall not be responsible for any incidental or consequential damages claimed to have occurred as a result of the sale, installation or use of any ResMed product. Some regions or states do not allow the exclusion or limitation of incidental or consequential damages, so the above limitation may not apply to you.















Need help?
Do you have a question about the Positive Airway Pressure Device VPAP IV and is the answer not in the manual?
Questions and answers