Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents
Troubleshooting

Subscribe to Our Youtube Channel
Summary of Contents for Fresenius Kabi Conox 2D
- Page 1 Conox® 2D Depth of Anesthesia Monitor Instructions for Use...
- Page 2 Abbreviations Alternating Current Analogue-Digital Converter Computed Tomography Direct Current Electroencephalogram Electromagnetic Compatibility Magnetic Resonance Imaging Printed Circuit Board Radio Frequency...
-
Page 3: Table Of Contents
Table of Contents GENERAL INFORMATION 1.1. Scope 1.2. Intended purpose 1.3. Clinical benefits 1.4. Indications for use 1.5. Contraindications 1.6. Contact address 1.7. Symbols 1.8. Disclaimer SAFETY 2.1. General safety instructions 2.2. Electromagnetic compatibility 2.3. Safety symbols 2.4. Packaging symbols DESCRIPTION 3.1. - Page 4 SETUP 4.1. Unpacking the Device 4.2. Inside the box 4.3. Installing for the first time SETUP MENUS 5.1. Operating modes 5.2. Recording mode Settings 5.2.1. Information screen 5.2.2. Language 5.2.3. Time 5.2.4. Bluetooth® 5.2.5. Trends and spectrogram settings 5.2.6. EEG settings 5.2.7.
- Page 5 8.2. Patient sensor 8.3. Skin Prep 8.4. Placement of electrodes 8.5. Connect patient cable & turn on 8.6. Impedance check 8.7. Event sequence / operation 8.8. Patient cable disconnected EXTERNAL POWER SUPPLY & BATTERY 9.1. External power supply 9.2. Battery 10.
- Page 6 15. SYSTEM AND ENVIRONMENT SPECIFICATIONS 15.1. Essential Performance 15.2. System specifications 15.3. Operation & shipment environment conditions 16. CYBERSECURITY RECOMMENDATIONS 17. DECLARATION OF CONFORMITY...
-
Page 7: General Information Scope
1. General Information 1.1. Scope These Instructions for Use (IFU) are relevant for the operation of the Conox Depth of Anesthesia monitor (the “Device”). Here you will find how to install, operate and maintain the Device. It is important that you read and understand the manual fully before using the Device. -
Page 8: Contraindications
Do NOT use in presence of CT, MRI, X-Ray machine. Conox is NOT intended to be used during defibrillation. 1.6. Contact address Manufacturer Fresenius Kabi AG Else-Kröner-Str. 1 61352 Bad Homburg Germany +49 (0) 6172 / 686-0 www.fresenius-kabi.com... -
Page 9: Symbols
1.7. Symbols Symbols used in this manual: Warning: A possible hazard may result in serious personal injury and/or damage to the product if instructions are not followed 1.8. Disclaimer Manufacturer reserves all rights. No part of this document may be reproduced or published, in any format without written consent of the Manufacturer. -
Page 10: Safety
Safety 2.1. General safety instructions The performance of the Conox device may be affected by the quality of the EEG signal. The quality of the contact between the Conox Sensor and the skin is the primary contributor to electrode-skin impedance. If the patient skin is not prepared as indicated or the sensor is positioned incorrectly, this may lead to artifacts and incorrect monitoring. -
Page 11: Electromagnetic Compatibility
Any cables have damaged insulation. Any liquid falls onto the Device. If this occurs, switch off the Device, disconnect the cables and follow the cleaning instructions in section 10. There are any signs of an electrical fault. There are any signs of mechanical faults or breakage. There is any mechanical damage or loss of rigidity. -
Page 12: Packaging Symbols
Do not use if damaged Input terminal connector packaging Recycle device according to recycling compliance Nonsterile packaging scheme Latex free Recyclable material Lot number Type BF equipment Battery symbol Wireless CE Mark and Notified Body Unique Device Identifier Number Name and address of the manufacturing facility (01) Product identifier GTIN (21) Product serial number... -
Page 13: Description
Description 3.1. System description Conox is a device designed to be used by clinicians during anesthesia and sedation procedures. A sensor placed on the patient’s forehead transmits the EEG signal to the analogue-digital converter (ADC); the ADC amplifies and digitizes this signal. - Page 14 • Main Unit – A tablet shaped unit containing PCBs and touch screen display. The unit can be attached to a pole clamp. • External Power Supply - An external medical grade DC power supply.
-
Page 15: Controls And Indicators
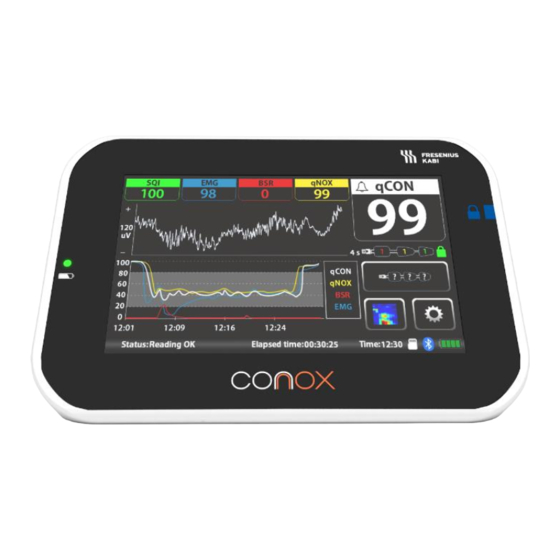
3.3. Controls and indicators The Device is controlled and interpreted via a touch sensitive screen: Indicators Controls qCON index EEG graph amplitude qNOX index EEG graph timescale Burst Suppression Ratio (BSR) Trends graph timescale Electromyogram index (EMG) qCON advisory status Signal Quality Index (SQI) Selected parameter trends EEG waveform/Spectrogram... -
Page 16: Qcon Index
3.4. qCON index The qCON index is a continuously processed EEG parameter that correlates with the patient's level of consciousness on a dimensionless scale of 0-99. Decreasing qCON index values correspond to the gradual loss of consciousness and a deepening of the level of anesthesia, so that qCON can be used to track the effects of certain anesthetics on the brain. -
Page 17: Qnox Index
3.5. qNOX index The qNOX index is a continuously processed EEG parameter that corresponds to the probability that a patient responds to noxious stimuli under general anesthesia or sedation, and it is reflected on a dimensionless scale of 0-99. qNOX Index Expected Clinical State Range Patient likely to respond to noxious... -
Page 18: Burst Suppression Ratio (Bsr)
3.6. Burst Suppression Ratio (BSR) With a range 0-100%, the Burst-Suppression Ratio (BSR) provides a measure of the percentage of flat EEG observed in a 30 second time frame. This parameter is helpful for detecting deep anesthesia. When the anesthesia is very deep, a specific pattern called Burst- Suppression is observed in the EEG. -
Page 19: Parameter Trend Graphs
The spectrogram and the EEG signal cannot be displayed at the same time. Spectrogram is accessed by pressing the “Spectrogram” button in the main Recording mode screen. Press the “EEG” button to display the EEG waveform again. 3.11. Parameter trend graphs qCON, qNOX, BSR and EMG parameters may be selected for graphing. -
Page 20: Sd Symbol
Error (See section 12) If the Bluetooth ® is disabled, no icon is displayed. Bluetooth ® is always enabled during Data Repository mode although no icon is displayed. 3.15. SD symbol The SD symbol shows correct functioning of the on-board memory. If a red cross is overlaid the symbol, it signifies a memory card error. -
Page 21: Device Status
3.18. Device Status The status can display the following messages: Artefact The Device has identified an artefact and rejected it. Status: Artefact An artefact is a received signal that is not consistent with normal EEG behavior according to the Device algorithms. Artefacts are rejected and not used in calculation of parameters. -
Page 22: Sensor Status
Deleting files The Device is erasing the selected file(s). Status: Deleting files Downloading The Device is sending the selected file to the PC via Bluetooth ® Status: Downloading Exploring files Stand by status until the user selects a file. Status: Exploring files Impedance test The impedance of the Patient Sensor is being checked. - Page 23 Check the validity date on The sensor the sensor connected to package. In case Conox is it is expired valid but it is replace the expired actual sensor for another one connected Replace the sensor has actual sensor reached the with a new one 24 hours of connected...
-
Page 24: Setup
Setup 4.1. Unpacking the Device DO NOT open or remove any covers of any of the delivered items due to the danger of electrical shock. DO NOT use any power source other than that supplied by the Manufacturer. If any parts delivered show signs of damage, do not use, and contact the Distributor immediately. - Page 25 Connect the External Power Supply. Turn the Device on. Adjust the settings of the Device. Refer to section 5 for information on how to do this. The equipment should not be installed next to any bag of liquids being used by the patient. Also, during cleaning the equipment should not be exposed to spills.
-
Page 26: Setup Menus
Setup menus 5.1. Operating modes Conox has two operating modes: Recording mode is default at turn on. In this mode the Device automatically starts recording a new case. Data Repository mode allows the user to explore, download, bookmark, review or delete recorded files. No monitoring can be performed while this mode is enabled. -
Page 27: Information Screen
Configurations selected by the user are saved for next session. 5.2.1. Information screen Press the “Information” button to see Conox firmware, hardware, software and serial number information. 5.2.2. Language To change the language, press the “Language” button. Conox is available in 20 languages: Czech Dutch Danish... -
Page 28: Time
Select one and press “Confirm”: 5.2.3. Time To set the current time and date, press the “Time” button. Set date and time and press “Confirm”. If time is changed while monitoring, data will be saved in a new file. 5.2.4. Bluetooth® To enable the Bluetooth ®... -
Page 29: Trends And Spectrogram Settings
When entering the Data Repository mode, Bluetooth® is automatically turned on and the symbol is no longer displayed. To pair Conox to another device: 1. Enable the Conox Bluetooth®. 2. Enable your device’s Bluetooth® and find the Conox. 3. Select the Conox through the serial number. If a password is required, please contact your local distributor to obtain this information. - Page 30 Trends selection Desired trends to be plotted may be selected by pressing the “Trends selection” button. Select the desired parameter/s (all displayed parameters may be selected) and press “Confirm”. Or press directly on the main screen trends legend. Trends graph and spectrogram timescale The trends graph and the spectrogram are synchronized in time, so they share the timescale.
-
Page 31: Eeg Settings
Trends grid To enable the grid in the trends graph, press the “Trends grid” button. Select graph grid “on” Or select graph grid “off”. 5.2.6. EEG settings When the spectrogram is displayed on the screen the EEG settings are disabled, so they cannot be adjusted. EEG graph amplitude EEG graph amplitude can be set by pressing the “EEG graph amplitude”... -
Page 32: Qcon Advisory Signal
EEG graph timescale EEG graph timescale may be set from the Setup menu by pressing the “EEG graph timescale” button. Select the timescale and press “Confirm”. Or press directly on the main screen the text label that indicates the current timescale. 5.2.7. - Page 33 The qCON advisory is designed with a sound pressure level between 45dB to 85dB. Use “+” and “-” buttons or move the horizontal bars to set the limits. Or to disable select “OFF”. When enabled, the area within the limits is shaded. In the image used as an example below the thresholds are set to 80 and 20.
-
Page 34: Data Repository
Advisory threshold levels are retained from the previous session. The qCON advisory settings can also be enabled from the qCON display box. 5.2.8. Data Repository To enter the Data Repository mode and work with recorded cases, press the “Data Repository” button in the Setup menu. Press the “Confirm”... -
Page 35: Data Repository Mode Settings
5.3. Data repository mode Settings Enter Data Repository mode and press the “Advanced setup” button. The following Advanced setup menu will appear: 5.3.1. File format Press the “File format” button to change the format of the file. Choose a format and press “Confirm”:... -
Page 36: Delete All Temporary Files
a) Default data: Downsampled and quantized (2 bytes/sample at 128Hz) data set stored taking up less memory and shorter download time. b) Full data: Full data set (3 bytes/sample at 1024Hz) stored. File sizes are bigger and download times longer. 5.3.2. - Page 37 Press “Confirm”:...
-
Page 38: Recording A New Case
Recording a new case To start recording a new case, press on the “Elapsed time” area in the status bar, and press the “Confirm” button. Conox will automatically start recording a new case in the following situations: Each time the Device is turned on. Each time the user leaves the Data repository mode to return to the Recording mode. -
Page 39: Data Repository Mode
Data Repository mode 7.1. Access Data Repository mode is activated by pressing the “Data Repository” button in the setup menu. When entering the Data Repository mode, Bluetooth® is automatically enabled and the monitoring of the current case is stopped. To exit the Data Repository mode, press "Back" and confirm the following message. -
Page 40: Recorded Cases
By default, Conox records and shows the Temporary files folder: User can access the Bookmarks folder by pressing the “Bookmarks” button. To access the Temporary files, press the “Temporary files” button. Each folder can store a maximum of 50 files displayed on several pages. The arrow buttons are used to navigate between screens. -
Page 41: Download A Case
7.3.1. Download a case To download a case, ConoxView must be installed on the user’s PC and the Conox and PC paired. In the ConoxView application press the “Download Data from Conox” tab, select the COM Port and press “Connect Conox”. Enter the Data repository mode in Conox, select a case and press “Download”. - Page 42 Once the download has finished a notification will be displayed: And the full case will be shown in the screen of your PC. If the download is not successful, the following message will appear. See section 12 for corrective actions. During the download, press “Pause”...
-
Page 43: Add A Case To Bookmarks
Press “Stop” to terminate the download. 7.3.2. Add a case to bookmarks The “Add to bookmarks” button is only enabled in the Temporary files folder Select a case and press the “Add to bookmarks” button. Press “Confirm”. When a case is bookmarked, it will be moved to the Bookmarks folder (not copied), i.e. - Page 44 A maximum of 50 cases may be saved in the Bookmarks folder. If the folder is full the selected case will not be bookmarked and the following message will appear: Space may be made in memory by deleting previous bookmarked cases.
-
Page 45: Review A Saved Case
7.3.3. Review a saved case To view the trends of a saved case, press on the "Review" button. The following message will appear: The case review will show the trends graph, including EMG, BSR, qNOX and qCON indices of the saved file. Press the “Back”... -
Page 46: Delete A Case
7.3.4. Delete a case Select a case and press the “Delete” button. The following message will appear to confirm deletion:... -
Page 47: Using The Device
Using the device 8.1. Positioning the Device The equipment should not be used adjacent to or stacked with other equipment. If this is necessary, the user should verify normal operation of the Device in the configuration that will be used. The Device requires special cautions regarding EMC. -
Page 48: Skin Prep
Do not use the Patient Sensor if it is past the date of expiry indicated on the packaging. Patient Sensor must not be used for more than 24 hours of continuous use. The Patient Sensor is a single use device and must be disposed of after use. -
Page 49: Placement Of Electrodes
It is important to take special care with patients with skin problems. Do not use alcohol to clean the skin as this may leave a film which increases the electrode impedance. 8.4. Placement of electrodes The Conox device is designed to work with Conox Patient Sensors only. -
Page 50: Connect Patient Cable & Turn On
If the adhesion or the contact of any of the electrodes is not enough after pressing around the Patient Sensor, use adhesive tape to press/stick the electrode to the skin. 8.5. Connect patient cable & turn on Connect the Patient Cable to the Main Unit. Connect the Patient Cable to the Patient Sensor. -
Page 51: Impedance Check
8.6. Impedance check The Device automatically checks the impedance of the Patient Sensor connections when it is turned on and every 15 minutes when in operation. Impedances below 10 kΩ are considered to be good, being the lower, the better. The impedance in kΩ will be displayed within the Impedance icon on the display. -
Page 52: Event Sequence / Operation
8.7. Event sequence / operation When the Device is turned on it will measure the impedance of the electrodes (approx. 5s) and then the sensor validity. The EEG trace will be displayed after. Simultaneously qCON, qNOX, EMG, BSR and SQI values will be displayed and the selected qCON, qNOX, BSR and EMG trends will then be displayed. -
Page 53: External Power Supply & Battery
Output power 10 W The Device must only be connected to a mains electrical supply with a protective earth. Only the external power supply provided by Fresenius Kabi may be used. The Device is electrically isolated from the mains supply. 9.2. - Page 54 The battery symbol indicates the state of charge: Battery Battery Battery full charging A battery symbol and the text “Recharge battery” will be displayed when the battery level is low. The battery must only be changed by technical personnel approved by Fresenius Kabi.
-
Page 55: Cleaning And Disinfection
10. Cleaning and disinfection 10.1. Service policy and rules Do not use cleaning or disinfection agents that contain the following substances as these aggressive agents may damage the plastic parts of the Device and cause the Device to malfunction: • Trichloroethylene •... -
Page 56: Cleaning Instructions
10.5. Cleaning instructions Prerequisites • The device is switched off and disconnected from the power supply. • The patient cable is disconnected from the Device and Conox Sensor. • The air is at room temperature (20 to 25°C). • The operator is wearing suitable protective equipment. Protocol 1. -
Page 57: Disinfection Instructions
10.6. Disinfection instructions Prerequisites • The cleaning protocol has been performed. • The Device is switched off. • The power cord and all other cables are unplugged. • The air is at room temperature (20 to 25°C). • The operator is wearing suitable protective equipment Protocol 1. -
Page 58: Adverse Effects
11. Adverse effects Patient Sensor may cause skin wounds due to allergy, irritability, or other kind of skin problems. If an artefact adversely affects the signal quality and decreases it, the qCON index and all other parameters will not be displayed. In case any adverse event is detected, Manufacturer and legal authorities shall be informed. -
Page 59: Troubleshooting And Diagnostics
12. Troubleshooting and Diagnostics 12.1. Conox Alarms All alarm conditions are classified as low priority based on the IEC 60601- 1-8 standard. Corrective Alarm message Possible causes actions Connect the Recharge battery power supply to The battery level Conox to continue is low. - Page 60 The sensor Replace the actual connected to sensor for a valid Invalid Sensor Conox is not valid one. If the sensor (Sensor contains used is already electronic valid, contact the identifier) Manufacturer Sensor time of use The connected exceeded. The sensor shall sensor has been Replace the actual be replaced.
- Page 61 electrocautery. It should resume normal operation after electrocautery is complete. The Device may EMG index be affected by indicates electrical EMC from other activity may be devices such as interfering with warming blankets. See section 14 for more information. Defective Patient Replace Patient Sensor Sensor...
-
Page 62: Troubleshooting & Diagnostics
12.2. Troubleshooting & Diagnostics Device status Possible causes Corrective actions If unintentional: - Connect external power supply - Replace external Operating on backup The Device is running from power supply cable battery battery - Replace external power supply - Defective Device, contact your Distributor Backup battery is totally Connect external power... -
Page 63: Service
Preventive maintenance must be performed by qualified and trained technical personnel in compliance with the technical manual and procedures. When replacing components, only use Fresenius Kabi parts. When using the device on the patient, no maintenance action must be performed. -
Page 64: Service Policy And Rules
Conox Sensor in general waste. For further information regarding waste processing regulations and dismantling, contact your local Fresenius Kabi sales representative. 13.5. Notification of serious incident Any serious incident that has occurred in relation to the Device should be reported to the manufacturer and the competent authority. -
Page 65: Electromagnetic Immunity
14. Electromagnetic immunity The Conox system is intended for use in the electromagnetic environment specified below. The user of the Conox must assure that it is used in an environment that meets these requirements. This section provides the appropriate specification tables for the Device as per IEC 60601-1-2. - Page 66 ± ± common common mode mode Voltage dips, < 5% UT (> < 5% UT (> Electrical mains power quality short 95% dip in should be that of a typical hospital interruptions UT) for 0.5 environment. voltage cycle. cycle. variations For short and long interruptions (<...
- Page 67 Conducted RF 3 Vrms 3 Vrms Portable mobile IEC 61000-4-6 150 kHz to 150 kHz to 80 communications equipment should 80 MHz be used no closer to any part of the Conox system, including cables, than the recommended separation distance calculated from equation...
-
Page 68: Recommended Separation Distances Between Portable And Mobile Rf Communications Equipment
14.1. Recommended separation distances between portable and mobile RF communications equipment The Device is intended to be used in the electromagnetic environment in which radiated RF disturbances are controlled. The user of the Device can help to prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Device as recommended below, according to the maximum output power of the communications equipment. - Page 69 15. System and environment specifications 15.1. Essential Performance The Device shall: • acquire EEG signals from the sensors (through the patient cable) in compliance with IEC 60601-2-26 • compute the qCON index • compute the qNOX index • compute an index to quantify the quality of the signals being received from the patient (SQI).
- Page 70 Indices update rate Selectable, high & low limit, audio and qCON advisory signal visual Total index update time 20 s Indices trend graphing Selectable, qCON, qNOX, EMG, BSR Lead off detection Continuous Automatic every 15mins, on request via Sensor impedance check panel button Technical specifications –...
- Page 71 Bluetooth Specifications Operating frequency range 2402 – 2480 MHz 1 Mbps UART Baud Rate: BR/EDR: up to 32 Kbytes/s LE: up to 7 Kbytes/s Maximum data rate 115200 bps UART Baud Rate LE: BR/EDR: up to 10 Kbytes/s LE: up to 6 Kbytes/s RX Sensitivity -90 dBm (BR/EDR), -92 dBm (LE) Output Power...
- Page 72 / operations / disposal of the devices. Medical devices must be deployed within a secure network perimeter to prevent access from unauthorized external system(s). Should you have concerns with connectivity to the CONOX device, contact your biomedical department or your Fresenius Kabi representative.
- Page 73 If you suspect a cybersecurity attack occurred or a vulnerability related to the Conox Device, please report this to your local Fresenius Kabi representative or submit a request to the Fresenius Computer Emergency Response Team (CERT, cert@fresenius.com).
- Page 74 17. Declaration of conformity The Device has passed the Immunity to emission of active RF surgical equipment test according to IEC 60601-1-1, IEC 60601-1-2, IEC 60601-1-6, IEC 60601-1-8 and IEC 60601-2-26. The Device has been produced in compliance with the Medical Device Regulation (EU) 2017/745, through the application of the following harmonized standards: UNE-EN 60601-1:2008 + ERR: 2008 + CORR: 2010 + A11:2012 (EN 60601-...
- Page 75 This document may not be reproduced in whole or in part without the written consent of Fresenius Kabi. Conox ® is a registered trademark in the name of Fresenius Kabi in selected countries. Made in France Revision date : AUGUST 2022...














Need help?
Do you have a question about the Conox 2D and is the answer not in the manual?
Questions and answers