Summary of Contents for Storz MASTERPULS ONE
- Page 1 PERATING ANUAL ® MASTERPULS DS.##### Part No. 27800 Published: 12.2020 Original language: German 29511 29511 0002...
- Page 2 0000000068...
- Page 3 This applies in particular to duplication, translation, microfilming as well as feeding and distribution in electronic systems. Manufacturer / Publisher STORZ MEDICAL AG Lohstampfestrasse 8 Telephone: +41 (0) 71 677 45 45 Postfach...
-
Page 4: Table Of Contents
Contents Introduction ..............General Safety Information. - Page 5 Service life ..............Trouble shooting .
-
Page 6: Introduction
Introduction This operating manual contains certain types of text design intended to assist you in comprehending the significance of the text based on its appearance. Instructions for actions • This text instructs you in how to operate your system correctly. •... - Page 7 CAUTION indicates that incorrect operation could lead to minor injuries. CAUTION The source of the danger is stated here. These are the possible consequences! ► The instructions for avoiding the danger are given here. NOTICE indicates that incorrect operation could lead to damage to the device. NOTICE The source of the danger is stated here.
-
Page 8: General Safety Information
The device is only to be used for the applications described in Chapter 2.1.1 I NDICATI • Only carry out treatments that have been approved by STORZ MEDICAL AG! Furthermore, the device is only allowed to be operated by trained personnel who fulfil the preconditions for operation in Chapter 2.2 P... -
Page 9: Safety During Treatment Of The Patient
The patient must not be under anaesthetic. Only perform treatments that have been approved by STORZ MEDICAL AG! The user is responsible for correctly positioning the handpieces and correctly selecting the treatment zone. - Page 10 Storage and transport Incorrect storage and transport can result in damage to the device and device failure. • Familiarise yourself with the ambient conditions for storage and transport in chap- ter 8.1 T ECHNICAL SPECIFICATIONS • Make sure that no cables are crushed or sheared. •...
-
Page 11: Principles
Principles Physical principles ® The MASTERPULS ONE is a compressed air operated ballistic pressure wave genera- tor. Kinetic energy is converted into sound energy. This acoustic pulse is transmitted into the tissue to be treated either directly or via an acoustic impedance adapter with the help of a gel. -
Page 12: Preconditions For Operation
Further training requirements vary from country to country. It is the operator's respon- sibility to ensure that the training meets the requirements of all applicable local laws and regulations. Further information about training in the operation of this device can be obtained from your STORZ MEDICAL dealer. 19 800 0002 12.2020... -
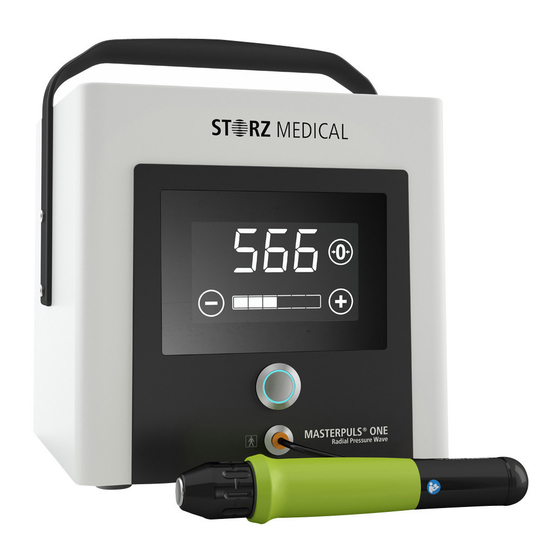
Page 13: System Description
System Description Control device with handpiece Handle Display Trigger button Handpiece connector Handpiece with coloured hand- piece cushion Pulse transmitter ® Fig. 3-1 Front MASTERPULS Ventilation slits Mains connector with mains switch and fuse ® Fig. 3-2 Rear MASTERPULS Scope of Supply ®... -
Page 14: Unpacking The Device
Unpacking the device • Remove the device and accessories from the packaging container. Proceed with extreme caution. • Check that all items are included in the packaging container and that they are not damaged. • Contact your supplier or the manufacturer/dealer immediately if any delivery items are missing or damaged. -
Page 15: Before Transport: Attaching The Transport Base Plate
3.3.2 Before transport: Attaching the transport base plate • To reattach the base plate, place the hook-and-loop tape underneath the base plate. • Lift the device onto the base plate so that the ribs on the transport base plate fit into the slits on the bottom of the device (F . - Page 16 Assemble the handpiece as follows: • Remove the guide tube and the projectile from the handpiece packaging. • Unscrew the shaft from the handpiece and pull it out of the handpiece handle. • Use the supplied open-end spanner for this purpose. •...
-
Page 17: Connecting The Electrical Power Supply
3.4.3 Connecting the electrical power supply • Connect the mains cable to the mains connector on the left rear of the device (see . 3-2 /1). • Insert the mains cable into the electrical socket. NOTICE ► Maintain a minimum distance between the device and the wall so that the mains plug can be pulled out without restrictions (disconnected from the power supply network) and the ventilation slits on the rear are not blocked. -
Page 18: Operation
Operation Switching on and off • Switch the control device on or off at the mains switch on the rear (see F . 3-2 ). The device is operated via the touchscreen display. Setting the intensity The display indicates the intensity (levels 1 – 6). •... -
Page 19: Reading Out The Device Data
Reading out the device data To enable the operating hours, total number of pulses and the software version to be read out, the device must be restarted. • Press during the restart The display will then show the operating hours, followed by the total number of pulses and then the software ver- sion. - Page 20 CAUTION If the handpiece is not positioned correctly, there is an impairment to health due to ineffective treatment! ► Define the treatment zone and make sure that the handpiece position always cor- responds to the treatment zone. ► Make sure that the treatment is only administered by users who meet the condi- tions in Chapter 2.2 P RECONDITIONS FOR OPERATION For safety reasons, using the device for applications other than those specified in chap-...
- Page 21 Coupling the handpiece Clean all parts which come into contact with the patient before and after each treat- ment. • Apply a sufficient amount of coupling gel to the patient’s skin in the treatment area and to the pulse transmitter. •...
-
Page 22: Cleaning, Maintenance, Overhaul
Cleaning, Maintenance, Overhaul Cleaning and overhaul ® Regular cleaning ensures perfect hygiene and operation of the MASTERPULS ONE. DANGER Electrical hazard Disconnect the device from the mains before starting any cleaning, mainte- nance or overhaul work. ► Disconnect the mains plug. The frequency of complete exterior cleaning depends on the frequency of use and what the device is used for. -
Page 23: Replacing The Pulse Transmitter
5.1.1 Replacing the pulse transmitter CAUTION Surface damage to the pulse transmitter due to wear and tear can lead to injuries. ► Replace the pulse transmitter after 1,000,000 pulses. ► Carry out the handpiece overhaul in good time as recommended in chapter 5.1.4 VERHAUL OF THE HANDPIECE ►... -
Page 24: Cleaning The Handpiece
Make sure that the cap parts of the pulse transmitters are screwed firmly in place and that the pulse transmitter screw cap is screwed firmly to the shaft. Check the screw connection of the pulse transmitter screw cap and cap parts during prolonged treatment phases. -
Page 25: Cleaning And Disinfecting The Pulse Transmitter
• Clean the guide tube using a brush. • Follow the instructions in reverse order to assemble the handpiece. When assembling the handpiece, always retighten the handpiece shaft using the sup- plied open-end spanner. It must no longer be possible to unscrew the shaft by hand. 5.1.3 Cleaning and disinfecting the pulse transmitter •... -
Page 26: Overhaul Of The Handpiece
5.1.4 Overhaul of the handpiece Due to the effects of friction, the handpiece components are continuously exposed to mechanical stress, which will cause minor wear. The handpiece should be overhauled about every 1,000,000 pulses. This can be done quickly and easily by the user of the device. All that is required is the overhaul kit, which includes all required wear parts. - Page 27 • Pull the tightly fitting guide tube out of the shaft. If necessary, use a thin metal rod or the supplied hexagonal spanner as a pulling tool by inserting it through the openings in the guide tube. • A corresponding fixture is provided in the handpiece handle to hold the projectile. To remove the projectile, hold the handpiece handle with its opening pointing down.
-
Page 28: Mains Fuse Replacement
• Insert the new projectile into the fitted guide tube. • Screw the shaft into the handpiece until finger-tight. • Tighten the shaft using the open-end spanner. It must no longer be possible to un- screw the shaft by hand. •... -
Page 29: Maintenance
Repair work on defective devices must only be carried out by personnel suitably autho- rised by the manufacturer. Only STORZ MEDICAL original parts from the manufacturer may be used for this purpose. The suitably authorised personnel can be from the STORZ MEDICAL AG or be representatives of its agencies and dealers. -
Page 30: Trouble Shooting
Trouble shooting Fault description Possible cause Corrective actions No pulse emitted 1. Leaks in hand- 1. Have the connection checked piece hose or and replaced, if necessary hose not properly connected 2. Dismantle the handpiece, turn 2. Guide tube in- the guide tube around stalled the wrong way round... -
Page 31: Accessories
Accessories Product description Article number Handpiece »SPARROW« grey 30064.0001 Handpiece »SPARROW« green 30064.0002 Handpiece »SPARROW« orange 30064.0003 ® MASTERPULS ONE overhaul kit 26894 Gel bottle 250 ml 22601 Mains cable CEE 3 m long 13455 Mains cable CH 3 m long 13448 Mains cable USA 16453... -
Page 32: Technical Specifications And Conformity
Technical Specifications and Conformity Technical specifications ® MASTERPULS Mains input voltage 100 VAC // 115 VAC // 230 VAC Mains frequency 50 / 60 Hz // 60 Hz // 50 - 60 Hz Mains fuse T 8 AH / 250 VAC Power consumption Max. -
Page 33: Type Plates
Type plates Fig. 8-1 230 V type plate Fig. 8-2 115 V type plate Fig. 8-3 100 V type plate Conformity with directives This medical product bears the CE mark in accordance with the Medi- cal Device Directive (MDD) 93/42/EEC. Conformity with standards This device complies with the applicable standards EN/IEC 60601-1, CAN/CSA-C22.2 No.601.1, UL Std. -
Page 34: Emc Guidelines And Manufacturer's Declaration
8.4.1 EMC guidelines and manufacturer's declaration Guidelines and manufacturer's declaration Emitted electromagnetic interference ® The MASTERPULS ONE model is intended to be used in the electromagnetic environment specified ® below. The customer or the user of the MASTERPULS ONE should ensure that it is used in such an environment. - Page 35 Guidelines and manufacturer's declaration Resistance to emitted electromagnetic in- terference ® The MASTERPULS ONE model is intended to be used in the electromagnetic environment specified ® below. The customer or the user of the MASTERPULS ONE should ensure that it is used in such an environment.
- Page 36 Guidelines and manufacturer's declaration Resistance to emitted electromagnetic in- terference ® The MASTERPULS ONE model is intended to be used in the electromagnetic environment specified ® below. The customer or the user of the MASTERPULS ONE should ensure that it is used in such an environment.
- Page 37 Recommended safety distances between portable and mobile RF communications equip- ® ment and the MASTERPULS ® The MASTERPULS ONE is intended for use in an electromagnetic environment in which radiated ® RF disturbances are controlled. The operator or the user of the MASTERPULS ONE an help prevent electro-magnetic interference by maintaining a minimum distance between portable and mobile RF ®...
-
Page 38: Certificates
Certificates Fig. 8-4 Declaration of conformity 19 800 0002 12.2020... -
Page 39: Symbols And Labels
Symbols and labels Label Meaning 230 V type plate 115 V type plate 100 V type plate You must read the operating manual WEEE symbol Earth* (* for this device version, it is a functional earth) UDI (Unique Device Identification): Barcode on the type plate for the machine-read- able identification of the medical product Tab. -
Page 40: Warranty And Service
Warranty and service NOTICE Modifications to the device, handpiece and the pulse transmitters are not permitted. Any unauthorised opening, repair or modification of the device by unautho- rised personnel will relieve the manufacturer of its liability and responsibility for safe system operation. This will automatically void the warranty even be- fore the end of the warranty period. -
Page 41: System Book
System Book Identification Inventory no.: Serial numbers: ® MASTERPULS Handpiece: Supplier Address: __________________________ Tel.: __________________________ __________________________ Fax: __________________________ __________________________ Email: __________________________ __________________________ Mobile: __________________________ Training of the operator Confirmation of instruction in the operation of the device according to Chapter 2.2.2 and about the instruction in the operation and cleaning RAINING OF THE OPERATOR of the handpieces.















Need help?
Do you have a question about the MASTERPULS ONE and is the answer not in the manual?
Questions and answers
Сколько ударов рекомендовано при пяточной шпоре на одну область?