Summary of Contents for Custo Med Custo spiro mobile
- Page 1 Operating Manual Spirometry custo spiro mobile custo diagnostic 5.8 CMA 0025 ∙ DK 2139 ∙ Version 1 ∙ 2022/10/25...
- Page 2 This operating manual may not be copied in its entirety or in part, duplicated in any form or by any means or translated into another language without the prior written consent of custo med GmbH. The manufacturer reserves the right to change the information in this operating manual without prior notice.
-
Page 3: Table Of Contents
2.7 Procedure of an examination................. 28 Software .................... 29 3.1 custo diagnostic program structure ............. 29 3.2 custo spiro mobile connection to the PC ............ 30 3.3 Calibrating custo spiro mobile ..............31 3.4 Performing the spirometry measurement ..........32 3.4.1 Reference measurement .............. - Page 4 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile 3.12 Confirming the evaluation ................60 3.13 Optional: Reporting with approval process ..........61 3.14 Ending the evaluation ..................62 3.15 Settings for spirometry ..................63 3.16 Error messages and solutions ................ 68 Hygiene .....................
-
Page 5: Safety
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Safety General notes 1.1.1 Symbols used in this Operating Manual Safety warning symbol, in case of dangerous situations with high and medium risk level, which may result in personal injuries IMPORTANT:... -
Page 6: Laws And Regulations Applicable To The Product
As either the operator or user of this device you should have read and understood the Operating Manual, in particular the safety instructions. Should serious incidents occur in connection with a custo med product, they must be reported by the user and/or patient to the manufacturer and the competent authority of the Member State in which the user and/or patient is established. -
Page 7: Disclaimer
Internet at: www.customed.de, under Contact, Distributors. You can also contact custo med GmbH directly at any time. We will be pleased to provide you with information about your authorised custo med distributor or contact your authorised custo med distributor and forward your queries. -
Page 8: Safety Installations And Safe Working
1.2.2 Ambient conditions, handling of the devices Emissions The custo med devices/systems are not suitable for use in rooms or areas with a risk of explosion. For installation and operation of the devices/systems, the EMC (electromagnetic compatibility) instructions in the Operating Manual must be observed. -
Page 9: Patient Safety
Do not remove battery compartment covers or other covers during use. USB cable Some custo med devices have a USB cable. This cable must not be kinked. Do not step on the USB cable, only roll up the cable loosely and allow it to hang freely during operation. - Page 10 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile that the electrode contacts do not come into contact with other conductive parts. All results achieved by automatic analysis and the resulting unconfirmed reports produced by the system must be considered as suggestions only. For diagnosis and therapy purposes it is essential that the results are checked and assessed by a qualified physician.
-
Page 11: System And Data Security
IT system or your hospital information system (HIS). custo med does not accept any responsibility for any changes to data in your IT system or your HIS which were made after the export from custo diagnostic. - Page 12 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Allocation of case and job numbers If case or job numbers are manually entered into the system or they are changed in the system, there is a risk of confusing patients and subsequent misdiagnosis if an incorrect entry is made by a user.
-
Page 13: Information On Emc (Electromagnetic Compatibility)
Information on EMC (Electromagnetic Compatibility) The use of other accessories, other converters and leads than those indicated, except for the converters and leads sold by custo med as spare parts for inner components, can lead to increased electromagnetic emissions or to a reduced electromagnetic immunity of the system. -
Page 14: Safety Instructions For Spirometry
(air humidity, temperature, etc.). Otherwise this may falsify the measurement data obtained. Only use bacterial and viral filters approved by custo med, such as custo spiro protect. Unsuitable filters may falsify the measurement data obtained. -
Page 15: Residual Risks Spirometry
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Residual risks spirometry CAUTION Cross-contamination and falsification of measured values due to soiling in the filter → Dispose of the custo spiro protect bacterial and viral filters after each examination. Do not reuse! -
Page 16: Hardware
The operator has to decide himself/herself whether to use custo spiro mobile for a patient in certain situations (e.g. in the case of disablement). The system is intended for use by trained specialist staff or physicians in clinics and medical practices. - Page 17 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Relative contraindications spirometry Due to increased myocardial stress or changes in blood pressure → Acute myocardial infarction within one week → Systemic hypotension or severe hypertension → Significant atrial/ventricular arrhythmia →...
-
Page 18: Symbols On The Devices And Packaging
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Symbols on the devices and packaging Manufacturer: custo med GmbH, Maria-Merian-Str. 6, 85521 Ottobrunn, Germany Order number/designation Lot designation Serial number Unique Device Identifier Medical device Date of manufacture (YYYY-MM, e.g., 2022-01) -
Page 19: Technical Data And System Requirements
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Technical data and system requirements custo spiro mobile Measuring sensor Differential pressure gauge with laminar element Display of measured values BTPS (Body Temperature Pressure Saturated) Measurement range 1.00l/s – 14.5 l/s... - Page 20 For proper operation it is necessary to use the operating system/software combinations tested and approved by custo med for the respective custo diagnostic version (also custo diagnostic server and client for custo diagnostic 5). These can be obtained from the authorised custo med dealer or directly from custo med.
- Page 21 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Recommended system requirements Computer Intel Core i3-CPU with HD graphics 4400, 4 GB RAM, 256 GB SSD or SSHD (for single-user workstations 2TB HDD), 1 GBit network connection (for single-user workstations),...
-
Page 22: Putting Out Of Operation, Storage, Transport, Disposal
Make sure that the storage location is dust-free, dry and away from direct sunlight. → After a long period of non-operation, the devices may only be used again if a technical safety check has been carried out by your authorised custo med distributor. Transport →... -
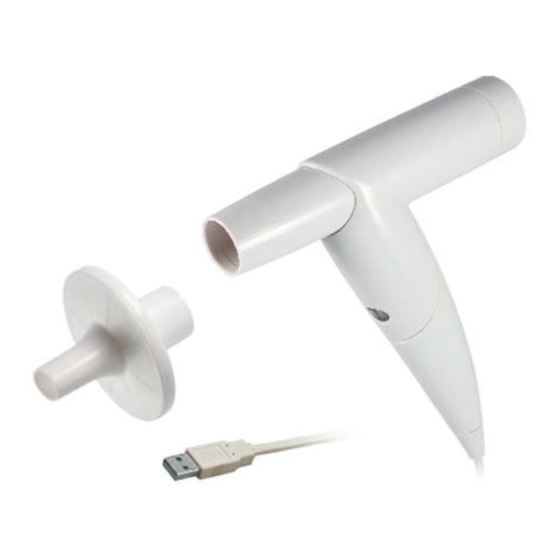
Page 23: Components For The Recording
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Components for the recording Fig. 2: custo spiro mobile part designation custo spiro mobile measuring unit spiro mobile custo spiro mobile adapter custo spiro protect bacterial and viral filter Calibration pump 3 liters Not shown →... -
Page 24: Device Operation
PC - connection via USB cable . The device is ready for operation. Fig. 3: custo spiro mobile function display CMA 0025 ∙ DK 2139 ∙ Version 001 ∙ 2022/10/25 ∙ www.customed.de... -
Page 25: Using The Bacterial And Viral Filters
Spirometry ∙ custo spiro mobile 2.6.2 Using the bacterial and viral filters The custo spiro mobile pulmonary function testing device must only be operated with custo spiro protect bacterial and viral filters custo spiro protect bacterial and viral filters are fitted on the mouthpiece before the measurement. -
Page 26: Disassembling And Assembling The Device
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile 2.6.3 Disassembling and assembling the device To disassemble the custo spiro mobile: → Press firmly on the release key → Pull the measuring unit upwards. → Pull the mouthpiece out of the measuring unit by rotating it slightly. -
Page 27: Notes On Calibration
2.6.4 Notes on calibration Calibration intervals The custo spiro mobile pulmonary function testing devices are pre-calibrated at custo med (10-stage calibration). Before putting the device into operation another calibration with custo diagnostic has to be carried out – in the process system and device are matched, taking the environmental conditions into account. -
Page 28: Procedure Of An Examination
Procedure of an examination Preparing for the measurement, custo spiro mobile → Check to make sure that custo spiro mobile is connected to the PC. → When the LED in the handle lights up, the device is ready for operation. -
Page 29: Software
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Software custo diagnostic program structure The custo diagnostic program is divided into three areas: User, Patient and Examination. This structure ensures that you can always recognise who (which user) is carrying out what type of examination with whom (which patient). -
Page 30: Custo Spiro Mobile Connection To The Pc
IMPORTANT: Prerequisite - custo diagnostic is installed on your PC and ready for operation. The custo med devices and components may only be connected to the PC after custo diagnostic has been installed. The required device drivers are installed on the PC via the custo diagnostic standard setup or by specific selection during the custo diagnostic setup. -
Page 31: Calibrating Custo Spiro Mobile
Calibrating custo spiro mobile IMPORTANT: Before using the device for the first time, a calibration must be carried out. → If you have not already done so, connect custo spiro mobile to the PC. → Assemble the devices as shown →... -
Page 32: Performing The Spirometry Measurement
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Performing the spirometry measurement INFORMATION: The steps necessary to perform and evaluate a spirometry measurement in custo diagnostic are shown without a surgery IT system or HIS connection. 3.4.1 Reference measurement Program start, calling the pulmonary function →... - Page 33 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Settings for the reference measurement 1) The preset predicted values author can be changed in the → Predicted value : the default setting is GLI (Global Lung Initiative) . The custo diagnostic settings.
- Page 34 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Overview of the measurement interface 1) LLN (Lower Limit of Normal) is the lower limit value, used to The predicted value that has been selected for the measurement series, assess “normal” or in this case GLI.
- Page 35 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Optional: Spirometry measurement with animation for children The “Animation for children” function is part of the To perform a measurement with animation for children, click on Options, “professional” software and not Animation.
- Page 36 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Performing a reference measurement 1) The number of resting breaths before the breathing → Put the nose clip on the patient. manoeuvre can be changed in → Click on Start. the settings. To do this, open →...
- Page 37 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Further functions within the reference measurement Fig. 16: Context menu of a measurement Fig. 17: Process control, reproducibility of a measurement. Repeating measurement → Click Repeat to perform another reference measurement. Up to six repeat measurements are possible.
- Page 38 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Ending and closing the measurement → With the default settings, the measurements are checked for reproducibility. If two reproducible measurements are available, a corresponding note appears and the measurement can be ended.
- Page 39 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Options during the reference measurement During the reference measurement, the following functions are available for editing and reporting in the Options menu: Print...: Print menu for compiling a printout Changing the Predicted values...
-
Page 40: Follow-Up Measurements: Spasmolysis And Provocation
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile 3.4.2 Follow-up measurements: Spasmolysis and provocation Spasmolysis and provocation are referred to as follow-up measurements. These types of measurements can only be performed following a reference measurement. Calling up a follow-up measurement →... - Page 41 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile INFORMATION on follow-up measurements: The setting options and operating elements of the follow-up measurements correspond to those of a reference measurement, e.g. Start, Repeat and Best Measurement. Performing a spasmolysis →...
-
Page 42: Unconfirmed Report
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile 3.4.3 Unconfirmed report IMPORTANT: All unconfirmed reports produced by the system should be considered as suggestions only. For diagnosis and therapy purposes it is essential that the results are checked and assessed by a qualified physician. - Page 43 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Displaying further automatic reports Via Options, Autom. Report, the following evaluations can be added to the report: → Standard, according to 70% rule for FEV1/FVC and 80% rule for IVC and FVC, →...
-
Page 44: Printing The Measurement
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile 3.4.4 Printing the measurement Printing with system settings: → Click on the Print button in the measurement interface. The system settings for the print pages of a spirometry measurement can be found in custo diagnostic on the screen page Examination, Spirometry, Settings, Print, Print pages. -
Page 45: Opening Evaluations
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Opening evaluations 3.5.1 Opening an evaluation via the evaluation search → To open the evaluation search right-click on the Patient button 1) The evaluation search can be configured in the custo →... - Page 46 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Advanced search, creating filter sets → The Advanced search is used to create filter sets and to quickly select search criteria (e.g., examination, properties, time period) . By setting Reference between the end certain search criteria, the search is narrowed down.
-
Page 47: Opening An Evaluation Via The Evaluation Main Menu
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile 3.5.2 Opening an evaluation via the evaluation main menu → Open the examination main menu via Examination, Spirometry. → Click on Show evaluation → The patient search screen appears. Select the patient whose evaluation Tip for entries in the patient you want to open. -
Page 48: Evaluation Structure
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Evaluation structure Structure of reference and spasmolysis measurements INFORMATION on the scope of functions: The Z-score, LLN, report assessment and the explanation according to clinical and occupational criteria are only available for measurements with the GLI predicted value. - Page 49 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Structure of provocation measurements INFORMATION on the scope of functions: The Z-score, LLN, report assessment and the explanation according to clinical and occupational criteria are only available for measurements with the GLI predicted value.
-
Page 50: Navigation In The Evaluation
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Navigation in the evaluation The buttons for opening the various evaluation screens are located at the bottom of the screen. By pressing one of the buttons, e.g., Comparison , the comparison view is opened and the name of the button changes to Evaluation (name of previous screen page). -
Page 51: Diagnostic Terms In The Evaluation
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Diagnostic terms in the evaluation Lower Limit of Normal (LLN) The green ranges in the bar diagram are defined by the predicted value (upper limit/right end) and LLN - Lower Limit of Normal (lower limit/left end) LLN is the lower limit value used to assess “normal”... - Page 52 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile achieved in comparison to the predicted value is entered as a percentage on the y-axis. The intersection of these values is marked with a cross. The marking crosses are in the colour of the measurement type.
-
Page 53: Evaluation Of The Reference And Spasmolysis Measurement
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Evaluation of the reference and spasmolysis measurement Fig. 31: Evaluation, overview Flow-volume curves (reference measurement: blue, spasmolysis: orange) Predicted values, in this case GLI Display of results for FEV, FVC and FEV1/FVC in a bar chart Table of measured values with predicted values, measured values, Z- score and deviations in percentage;... -
Page 54: Evaluation Of A Provocation Measurement Series
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile 3.10 Evaluation of a provocation measurement series Fig. 32: Evaluation, provocation Graphical representation of all the measurements of the measurement series with the selected measured value displayed as a bar, here IVC... -
Page 55: Further Screens Of An Evaluation
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile 3.11 Further screens of an evaluation History control (only for reference and spasmolysis evaluations). This screen is opened via the Process Control button. The process control is used to compare a series of reference and/or spasmolysis measurements in order to check the quality of the patient's cooperation well as the plausibility of the results. - Page 56 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Options menu, Repeatability In order to make a statement on the quality and plausibility of a measurement series, the FEV1 values of a measurement series and the FVC values of a measurement series are compared with each other.
- Page 57 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Comparison Fig. 34: Evaluation, comparison The Comparison function (bottom left in the evaluation overview) can be used to compare the open evaluation with another evaluation of the patient. The comparison can also be called up via the Spirometry main menu with Show Comparison.
- Page 58 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Trend Fig. 35: Evaluation, trend view The trend view is opened via the Spirometry main menu with Show Trend or in the evaluation via Options, Trend. The trend view is used to display developments over a longer period of time.
- Page 59 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Z-Score Trend Fig. 36: Evaluation, Z-Score Trend The Z-score trend can be called up in the trend view of an evaluation (Open evaluation, Options, Trend). The Z-score values of a measured value are plotted as a trend over time (y-axis: Z-score, x-axis: date).
-
Page 60: Confirming The Evaluation
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile 3.12 Confirming the evaluation Unconfirmed report and report To open the unconfirmed report, right-click on the evaluation interface. In the context menu select Report. Enter your data in the text field . -
Page 61: Optional: Reporting With Approval Process
The approval process must be activated in the Settings and in the custo service center for each user and project. The user rights must be set to match the workflow. Contact your authorised custo med dealer or custo med. INFORMATION:... -
Page 62: Ending The Evaluation
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile 3.14 Ending the evaluation Click on End (bottom right) in the evaluation. The End dialogue opens. The status of an evaluation is defined here. Assigning properties (status of the evaluation) in the End dialogue makes it easier to find evaluations in the evaluation search. -
Page 63: Settings For Spirometry
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile 3.15 Settings for spirometry Configuring the printout On the Examination, Spirometry, Settings, Print, General screen page, you define which print pages are printed when the Print button is pressed. In the “Print sequence...”... - Page 64 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Parameters for the spirometry measurement On the Examination, Spirometry, Settings, Diagnostic , Parameter screen page, various parameters can be set for the measurement: → Predicted values and area of validity: Define which predicted values should be proposed by default for children and adults .
- Page 65 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Display of measured values in the software interface and in the printout On the Examination, Spirometry, Settings, Diagnostic , Measur. Display screen page, you can set for each set of predicted values which measured values are to be displayed in the software interface and in the printout (if a different display from the default setting is desired).
- Page 66 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Procedures and functions for the spirometry measurement These settings can be found on the Examination, Spirometry, Settings, Menu/Functions , Menu/Functions screen page. → Measurement units for the environmental data : In the “Environmental data”...
- Page 67 The function supports the correct use of the custo spiro mobile measuring device as well as its maintenance and care in order to permanently ensure the quality of the measurements. The review mechanism examines the best reference measurements from five consecutive patients.
-
Page 68: Error Messages And Solutions
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile 3.16 Error messages and solutions Error message: Spirometry device not ready for use → Confirm the error message, close the spirometry software if necessary. → Disconnect the USB plug of the spirometry testing device from the PC. -
Page 69: Hygiene
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Hygiene Important notes Only use cleaning agents and disinfectants recommended by custo med. Unsuitable agents may damage the device. The handle of the device must not be immersed in liquids. The measuring unit... -
Page 70: Hygienic Reprocessing
. For infectious patients see 4.4 Disposal of contaminated consumables, p. → Disinfect custo spiro mobile and all associated components/parts that have come into contact with the patient from the outside, see 4.2.3 Wipe disinfection, after each examination, p. →... -
Page 71: Disassembling The Device For Cleaning And Disinfection
→ Make sure that the sealing ring in the handle is not damaged. → If possible, clean or disinfect immediately after use. Fig. 48: Disassembling custo spiro mobile CMA 0025 ∙ DK 2139 ∙ Version 001 ∙ 2022/10/25 ∙ www.customed.de... -
Page 72: Wipe Disinfection, After Each Examination
No moisture may get into the handle (e.g. via the sensor). → When all parts of the custo spiro mobile device have been cleaned, disinfected and dried, reassemble the device. → The release button must engage with a click when placing the measuring unit. -
Page 73: Instrument Disinfection, Weekly Or After 100 Examinations
No moisture must get into the handle (e.g., via the sensor). → When all parts of the custo spiro mobile device have been cleaned, disinfected and dried, reassemble the device. The release button must engage when the measuring unit is put on. -
Page 74: Recommended Cleaning Agents And Disinfectants
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Recommended cleaning agents and disinfectants Wipe disinfection: → Meliseptol® Wipes sensitive (B.Braun) → Meliseptol® Foam pure (B.Braun), use a soft, lint-free cloth for this purpose. → Observe the manufacturer's instructions! Instrument disinfection: →... -
Page 75: Disposal Of Contaminated Consumables
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Disposal of contaminated consumables Concerns custo spiro protect bacterial and viral filters and nose clips. In principle, the country-specific local rules and regulations apply. For the Federal Republic of Germany as follows: According to the Waste Catalogue... -
Page 76: Appendix
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Appendix Abbreviations of the spirometry measured values Abbreviation Unit Description AFEV l2/s Area under flow volume curve l/min Breathing frequency at rest (Breathing Frequency) Expiratory reserve volume FEF25%FVC = MEF75%FVC FEF25-75%... - Page 77 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Abbreviation Unit Description FIV2/VC Relative 2 second capacity of VC (inspirat.) in percent FIV3 3 second capacity (forced inspiratory volume in 3 seconds) FIV3/VC Relative 3 second capacity of VC (inspirat.) in percent...
-
Page 78: Calculation Tables For Predicted Values
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Calculation tables for predicted values The predicted values define their areas of validity using age, height, weight, ethnicity etc. A suitable predicted value for the measurement is allocated to the patient according to his/her data. The standard setting for children and adults is GLI. - Page 79 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Multicèntrico di Boys Girls Barcelona H = 85 - 180 cm | A = 6 - 20 years H = 85 - 180 cm | A = 6 - 20 years 0.02800 * H + 0.03451 * G + 0.05728 * A - 3.21...
- Page 80 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Crapo Women H = 150 - 220 cm | A = 18 - 120 years H = 150 - 220 cm | A = 18 - 120 years 6.00 * H - 0.0214 * A - 4.650 4.91 * H - 0.0216 * A - 3.590...
- Page 81 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Cherniak Women H = 150 - 190 cm | A = 15 - 79 years H = 150 - 190 cm | A = 15 - 79 years [ml] 47.6 * H - 14 * A - 3180 30.7 * H - 15 * A - 1310...
- Page 82 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Ulmer Women H = 150 - 195 cm | A = 15 - 75 years H = 150 - 195 cm | A = 15 - 75 years G = 40 - 170 kg...
- Page 83 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Hankinson Boys Caucasian/Asian H = 75 - 180 cm | A = 4 - 19 years FEV1%VC 88.066 + (-0. 2066 * A) FEV1 0.7453 + (-0.04106 * A) + (0.004477 * A * A) + (0.00014098 * H * H) (0.7453 + (-0.04106 * A) + (0.004477 * A * A) + (0.00014098 * H * H)) * 37.5...
- Page 84 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Hankinson Girls Caucasian/Asian H = 75 - 180 cm | A = 4 - 17 years FEV1%VC 90.809 + (-0.2125 * A) FEV1 -0.8710 + (0.06537 * A) + (0.00011496 * H * H) (-0.8710 + (0.06537 * A) + (0.00011496 * H * H)) * 37.5...
- Page 85 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Hankinson Men Caucasian/Asian H = 150 - 200 cm | A = 20 - 120 years FEV1%VC 88.066 + (-0.2066 * A) FEV1 0.5536 + (-0.01303 * A) + (-0.000172 * A * A) + (0.00014098 * H * H) (0.5536 + (-0.01303 * A) + (-0.000172 * A * A) + (0.00014098 * H * H)) * 37.5...
- Page 86 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Hankinson Women Caucasian/Asian H = 140 - 200 cm | A = 18 - 120 years FEV1%VC 90.809 + (-0.2125 * A) FEV1 0.4333 + (-0.00361 * A) + (-0.000194 * A * A) + (0.00011496 * H * H) (0.4333 + (-0.00361 * A) + (-0.000194 * A * A) + (0.00011496 * H * H)) * 37.5...
- Page 87 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile ECCS Children/Quanjer Boys Girls H = 75 - 180 cm | A = 4 - 17 years H = 75 - 180 cm | A = 4 - 17 years 0.95 * H 0.
- Page 88 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Kainu (Finland) Men and women Age = 18 - 83.99 years, ethnicity: none The predicted values are calculated for: FEV1, FVC, FEV1FVC, MEF75, MEF50, MEF25, MMEF (FEF25-75), PEF, FEV6, FEV1FEV6. The predicted values are calculated depending on gender, height and age.
- Page 89 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile References for predicted values 1) Austrian reference values – sources: Skriptum SPIROMETRIE Der Österreichischen Gesellschaft für Pneumologie; Prepared by the members of the Working Group for Clinical Respiratory Physiology, Standardization and Assessment.
-
Page 90: Keyboard Navigation And Shortcuts
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Keyboard navigation and shortcuts Use the quick links in the main navigation, the keyboard navigation and the keyboard shortcuts to enable fast and convenient working. Quick links in the main navigation Left click →... - Page 91 Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Keyboard shortcuts General shortcuts Enter Confirm Tabulator Cursor jumps to next input field (patient menu) Ctrl I Program information Ctrl H User main menu Ctrl P Patient main menu Ctrl U...
-
Page 92: Manufacturer's Declaration Regarding Emc
IEC 61000-3-2 Not applicable Voltage fluctuations/flickers IEC 61000-3-3 Not applicable Manufacturer’s declaration – electromagnetic immunity custo spiro mobile meets the test levels specified here. Phenomenon EMC standard / test method IMMUNITY TEST LEVEL Static electricity discharge (ESD) IEC 61000-4-2 ± 8 kV contact discharge ±... - Page 93 Recommended protective distances between portable and mobile RF telecommunication devices and custo spiro mobile custo spiro mobile is designed for use in an electromagnetic environment in which the RF transients can be controlled. The user can help avoid electromagnetic interference by maintaining the minimum distance between...
-
Page 94: Ec Declaration Of Conformity
Spirometry ∙ custo spiro mobile EC Declaration of Conformity Simplified declaration of conformity custo spiro mobile complies with the requirements of the Medical Device Directive 93/42/EEC or Medical Device Regulation (EU) 2017/745 and Directive 2011/65/EU. Hereby custo med declares that the radio device type(s) custo cardio 300 BT;... -
Page 95: Product Components And Accessories
Safety Hardware Software Hygiene Spirometry ∙ custo spiro mobile Product components and accessories Description Product designation Part no. Quantity/pc. custo spiro mobile 11051 Description Accessories Part no. Quantity/pc. measuring unit spiro mobile 11035 custo spiro mobile adapter 11034 custo spiro protect bacterial and viral filter... -
Page 96: List Of Figures
Fig. 4: Attaching the custo spiro protect bacterial and viral filter Fig. 5: Disassembling custo spiro mobile Fig. 6: custo diagnostic main menu Fig. 7: Settings screen for custo spiro mobile Fig. 8: Calibration pump with custo spiro mobile Fig. 9: Calibration screen Fig. - Page 97 GmbH Maria-Merian-Str. 6 85521 Ottobrunn, Germany Phone: +49 (0) 89 710 98-00 Fax: +49 (0) 89 710 98-10 info@customed.de www.customed.de...













Need help?
Do you have a question about the Custo spiro mobile and is the answer not in the manual?
Questions and answers