KaVo SURGmatic S201 XL Instructions For Use Manual
Hide thumbs
Also See for SURGmatic S201 XL:
- Instructions for use manual (28 pages) ,
- Instructions for use manual (28 pages) ,
- Instructions for use manual (28 pages)
Summary of Contents for KaVo SURGmatic S201 XL
- Page 1 Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333...
- Page 2 Distributed by: Manufacturer: KaVo Dental Technologies, LLC Kaltenbach & Voigt GmbH 11727 Fruehauf Drive Bismarckring 39 Charlotte, NC 28273 USA D-88400 Biberach Phone: 847 550 6800 www.kavo.com Fax: 847 550 6825...
-
Page 3: Table Of Contents
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 Table of contents Table of contents 1 User instructions ........................ 4 2 Safety..........................7 2.1 Infection hazard ......................7 2.2 Technical condition......................7 2.3 Accessories and combination with other equipment ............7 2.4 Qualification of personnel.................... -
Page 4: User Instructions
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 1 User instructions 1 User instructions Dear User Congratulations on purchasing this KaVo quality product. By following the in- structions below you will be able to work smoothly, economically and safely. - Page 5 Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 1 User instructions Information on the packaging Material number Serial number Legal Manufacturer CE mark according to Medical Devices Directive EC 93/42 Please note the electronic instructions for use...
- Page 6 Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 1 User instructions NOTICE In cases which – if not prevented – could lead to material damage. 6 / 26...
-
Page 7: Safety
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 2 Safety | 2.1 Infection hazard 2 Safety The instructions for use are a component of the product and must be read care- fully prior to use and be accessible at all times. -
Page 8: Qualification Of Personnel
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 2 Safety | 2.4 Qualification of personnel ▶ Only use accessories that have been approved for combination with the product by the manufacturer. ▶ Do not make any modifications to the device unless these have been ap- proved by the manufacturer of the product. -
Page 9: Description Of The Device
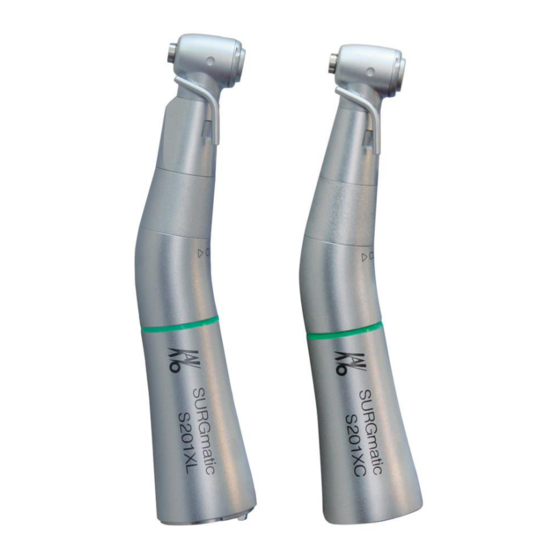
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 3 Description of the device | 3.1 Purpose – Proper use 3 Description of the device SURGmatic S201 XL SURGmatic S201 XL (Mat. no. 1.010.2332) SURGmatic S201 XC SURGmatic S201 XC (Mat. -
Page 10: Transportation And Storage Conditions
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 3 Description of the device | 3.3 Transportation and storage conditions Usable with surgical drill bits or burs with inner cooling or surgical tools with a hexagon head socket at the shaft. -
Page 11: Start Up And Shut Down
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 4 Start up and shut down | 4.1 Checking the amount of water 4 Start up and shut down WARNING Hazard from non-sterile products. Infection hazard for dentist and patient. -
Page 12: Operation
It is not permissible to combine the unit with other heads / shanks. Note The head of the SURGmatic S201 XL / XC should be taken off the shank only for purposes of reprocessing. WARNING Detachment of the medical device during treatment. -
Page 13: Attaching The Contra-Angle Handpiece To Motor Coupling
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 5 Operation | 5.3 Attaching the contra-angle handpiece to motor coupling 5.3 Attaching the contra-angle handpiece to motor coupling WARNING Detachment of the medical device during treatment. -
Page 14: Removing The Milling Tool Or Diamond Grinder
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 5 Operation | 5.6 Removing the milling tool or diamond grinder CAUTION Injury from using worn cutters or grinders. Cutters or grinders could fall out during treatment and injure the patient. -
Page 15: Troubleshooting
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 6 Troubleshooting | 6.1 Check for malfunctions 6 Troubleshooting 6.1 Check for malfunctions CAUTION Heating of the product. Burns or product damage from overheating. ▶ Do not use the product if it is irregularly heated. - Page 16 Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 6 Troubleshooting | 6.2 Troubleshooting ▶ Use the nozzle pin (Mat. no. 0.410.0931) to free the water passage on the spray clip on both sides. 16 / 26...
-
Page 17: Reprocessing Steps In Accordance With En Iso 17664
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 7 Reprocessing steps in accordance with EN ISO 17664 | 7.1 Preparation at the site of use 7 Reprocessing steps in accordance with EN ISO 17664 7.1 Preparation at the site of use WARNING Hazard from contaminated products. -
Page 18: Manual Reprocessing
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 7 Reprocessing steps in accordance with EN ISO 17664 | 7.3 Manual reprocessing For validated internal cleaning of the spray clip and spray tube in the cleaning and disinfecting device, preliminary non-fixing cleaning is required. -
Page 19: Automated Internal And External Cleaning And Internal And External Disinfection
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 7 Reprocessing steps in accordance with EN ISO 17664 | 7.5 Care products and systems - Servicing NOTICE Never disinfect the handpiece with chloride-containing products. Malfunction and material damage. -
Page 20: Care With Kavo Spray
▶ Remove the tool from the medical device. ▶ Cover the medical device with the KaVo CLEANpac bag, and place it on the corresponding care adapter. ▶ Press the spray key for 1 to 2 seconds. -
Page 21: Packaging
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 7 Reprocessing steps in accordance with EN ISO 17664 | 7.6 Packaging Servicing the clamping chuck KaVo recommends servicing the chuck system once a week using the chuck servicing program integrated in the device. -
Page 22: Storage
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 7 Reprocessing steps in accordance with EN ISO 17664 | 7.8 Storage NOTICE Contact corrosion due to moisture. Damage to product. ▶ Immediately remove the product from the steam steriliser after the steril- isation cycle. -
Page 23: Tools And Consumables
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 8 Tools and consumables 8 Tools and consumables Available from dental suppliers. Material summary Mat.No. Spray head INTRA (KaVo Spray) 0.411.9911 Service coupling for heads 0.411.7941 (QUATTROcare) Spray clip 1.002.3377... -
Page 24: Terms And Conditions Of Warranty
Instructions for use SURGmatic S201 XL - 1.010.2332 SURGmatic S201 XC - 1.010.2333 9 Terms and conditions of warranty 9 Terms and conditions of warranty The following warranty conditions apply to this KaVo medical device: KaVo provides the end customer with a warranty of proper function and guar-...











Need help?
Do you have a question about the SURGmatic S201 XL and is the answer not in the manual?
Questions and answers