Subscribe to Our Youtube Channel
Summary of Contents for Enraf Nonius Endomed 182
- Page 1 Endomed 182 EN109-1634750-40 IFU September 21, 2020 Instructions for use Page 1 of 52 EN109-1634750-40 IFU...
-
Page 2: Table Of Contents
Table of contents Introduction ..............................3 Symbols ................................7 Device components ............................9 Package contents ............................13 Installation ..............................15 Intended use and intended user ......................16 Indications ..............................17 Contra-indications ............................18 Precautionary instructions ........................20 10 Operation ................................ 23 11 Application information.......................... -
Page 3: Introduction
This manual has been written for the owners and operators of the Endomed 182. In order to maximize the use, efficiency and lifespan of your unit, please read this manual thoroughly and become familiar with the controls as well as the accessories before operating the device. - Page 4 For Endomed 182 Type 2 and 3 the electrical stimulation can thus be applied to the skin via surface electrodes: flexible rubber electrodes, self-adhesive electrodes or vacuum electrodes (Type 3 only), with the objective to stimulate specific types of nerve fibers.
- Page 5 Interferential currents are all AC currents without any residual DC components. Several variations of the interferential current type are known, of which the following are available in the Endomed 182 Type 2 and 3: 2-pole, 4-pole and 4-pole with isoplanar vector. The two-pole method...
- Page 6 The four-pole method (Classic Interferential) With this therapy method four electrodes are used and two non-modulated currents are generated. The frequency of one channel is fixed at the carrier frequency, while the other channel has a variable frequency, based on the Beat frequency and Frequency Modulation settings.
-
Page 7: Symbols
2 Symbols Symbol used Description Follow the instructions in the Instructions for Use. It is important that you read, understand and observe the precautionary and operating instructions General Prohibition Sign. Prohibition is used to mean “You MUST NOT” Warning or Caution: Indicates a hazardous situation which, if not avoided, could result in: Death or serious injury to the patient (or) b. - Page 8 Connection electrode cable electrotherapy Connections vacuum cables electrotherapy “ON” (power) “OFF” (power) CE Mark along with number indicates conformity with European Council of Directive concerning Medical Devices and this device is under the direct supervision of the Notified Body. Page 8 of 52 EN109-1634750-40 IFU...
-
Page 9: Device Components
3 Device components Endomed 182 Type 1 Endomed 182 Type 2 Page 9 of 52 EN109-1634750-40 IFU... - Page 10 Endomed 182 Type 3 Page 10 of 52 EN109-1634750-40 IFU...
- Page 11 Return electrode connection cable Electrode holder Handpiece for needle holder Connection A Connection for Vacuum Module (Endomed 182 Type 3 / Footswitch (Endomed 182 Type 1) Connection B Connection for Current Module Current Module Current Module with connection for adhesive and rubber electrodes.
- Page 12 Parts Description Numbered Description Purpose Part [21] Connection for water reservoir This connection is used for connecting the water reservoir. No vacuum can be created unless the water reservoir is connected. The water reservoir ensures that any excess water from the sponges is collected, and cannot be blown out through the pump.
-
Page 13: Package Contents
3444080 Electrode holder 3444582 Blank earth electrode 3444677 DC reference cable Standard accessories Endomed 182 Type 2 and Endomed 182 Type 3 1634750 CD-ROM with Instructions For Use (PDF on CD-ROM) 1634751 Information Booklet 3444357 Power cord 250V/2.5 A, L=2.5 m, black... - Page 14 Sponges Ø 30 mm, set of 4 (for vacuum electrodes Ø 30 mm) Vacuum lead hoses 3444507 Vacuum lead hose, red 3444508 Vacuum lead hose, black Ordering information For the ordering data of the Endomed 182 and accessories we refer to the website www.enraf-nonius.com. Page 14 of 52 EN109-1634750-40 IFU...
-
Page 15: Installation
• Unpacking The Endomed 182 is specially packaged for transport in a single pack complete with filling which has been specifically designed for safe transportation and storage. To remove the equipment from the pack, place the box on a smooth, flat surface. -
Page 16: Intended Use And Intended User
2. through the release of the body’s endogenous opioids by stimulation of the small diameter afferent and motor fibers Intended user The Endomed 182 is intended to be used, and shall only be used, by or under the supervision of professional users in the field of physical medicine and rehabilitation at professional healthcare facilities. -
Page 17: Indications
Pubalgia (groin pain) • Hamstring tendinopathy • Achilles tendinopathy • Fasciitis Plantaris / Heel Pain • Indications Endomed 182 Type 2 and 3 TENS Relief of acute pain Postoperative pain • Abdominal Thoracic p.o. orthopedic pain Labour pain and post-labour pain •... -
Page 18: Contra-Indications
8 Contra-indications The Endomed 182 MUST NOT be used for the below mentioned symptoms or medical conditions. Contraindications Endomed 182 Type 1 Contraindications for use Infectious arthritis • Heart disease / cardiac arrhythmia • Pregnancy • Skin ulcers and other skin infections •... - Page 19 Contraindications Endomed 182 Type 2 and 3 Absolute contraindications: Undiagnosed pain • Active implants (incl. cardiac pacemakers and implantable cardioverter defibrillators - ICDs) • Tissue bleeding (haemorrhage) • Patients who do not comprehend the practitioner’s / physical therapist’s instructions or who are •...
-
Page 20: Precautionary Instructions
9 Precautionary instructions If the use of this device may have caused or contributed to an undesirable event such as death or serious injury to the user, the manufacturer AND the competent authority of the Member State MUST be notified immediately! This device should be kept out of the reach of children. - Page 21 Maintenance shall not be performed on the equipment when equipment is in use or connected to a patient. It is recommended to have the Endomed 182 checked yearly by authorized personnel of Enraf-Nonius or by its authorized distributor. Medical electrical devices such as the Endomed 182 are subject to special precautions with regard to electromagnetic compatibility (EMC) and must be installed and commissioned in accordance with the EMC advice given in the instructions for use.
- Page 22 Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm to any part of the Endomed 182, including cables specified by the manufacturer. Otherwise degradation of the performance of this equipment could result.
-
Page 23: Operation
Set up Do not use the device after extreme temperature fluctuations! Frequently Used Functions Selecting the Clinical Protocols (only applies to Endomed 182 Type 2 and 3), Favorite and Manual Operation buttons in the touch screen. Switch On Switch the unit On by pushing the On/Off Button at the back of the unit. - Page 24 Select System Settings in the Home Menu, to adjust various settings, check the software version or restore the device to factory defaults. The options Acoustic Feedback, Buzzer Tone and Buzzer Duration are only available in the Endomed 182 Type 2 and 3. Set Language 1) Select “System Settings”...
- Page 25 2) Push BACK to return to the previous menu Acoustic Feedback Endomed 182 Type 2 and 3: Select this option to turn on or off audio signals at the end of treatment. 1) Select “Acoustic Feedback”...
- Page 26 Buzzer Tone and Buzzer Duration Endomed 182 Type 2 and 3: Buzzer Tone provides access to Buzzer Duration provides 7 different tunes to signal the access to 7 different intervals 1) Select “Buzzer Tone” or end of a treatment. to signal the end of a “Buzzer Duration”...
- Page 27 Manual Operation Endomed 182 Type 1 The Endomed 182 Type 1 has one output channel. See chapter 11 for connection of electrodes. 1) Select “Manual Operation” in Select (K) to set treatment time 2) Set treatment time the Home Screen...
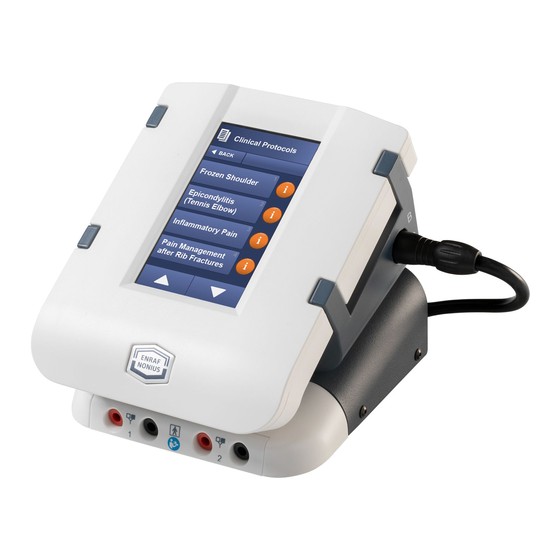
- Page 28 Clinical Protocols For the Endomed 182 Type 2 and 3 a list of common electrotherapy treatments can be found under Clinical Protocols. These evidence-based protocols have been created by professional and expert operators after years of experience in the field.
- Page 29 7) The parameter screen shows 10) Adjust the intensity level via 12) The protocol will start after all parameters at a glance the buttons setting the intensity level 8) The pre-set clinical protocol 11) Push OK to confirm and 13) The protocol can be parameters can be adjusted return to the previous screen...
- Page 30 With Manual Operation personal parameter settings can be defined for electrotherapy. The Endomed 182 Type 2 and 3 has two independent output channels. To select the output channel, touch the channel tab on the bottom of the screen. See chapter 11 for connection of electrodes.
- Page 31 Setting Parameters: Burst Frequency 1) Select Burst Frequency (C) 2) Set Burst Frequency Option: 1 Hz / 2 Hz / 4 Hz 3) Push OK to confirm and return to the parameter screen Setting Parameters: Modulation Frequency 1) Select Modulation Frequency (D) 2) Adjust Modulation Frequency via the ...
- Page 32 Setting Parameters: Modulation Time 1) Select Modulation Time (E) 2) Set Modulation Time Option: 1-1 seconds 6-6 seconds 1-30 seconds 3) Push OK to confirm and return to the parameter screen Setting Parameters: Treatment Time 1) Select Treatment Time (F) 2) Set treatment time via the ...
- Page 33 Setting Parameters: Vacuum Module With the Endomed 182 Type 3 it is possible to use vacuum electrodes 1) Select Vacuum (G) 2) Set vacuum parameters Option: pulsed mode 1 Hz / pulsed mode 2 Hz / continuous 3) Adjust the pressure of the vacuum cups via the ...
- Page 34 Setting Parameters: Balance Control For 4P Interferential the balance control option is available after Intensity has been set. With balance control the intensity of Channel 1 relative to Channel 2 can be increased or decreased. An increase of the intensity of Channel 1 will automatically decrease the intensity of Channel 2 and vice versa.
- Page 35 4) Enter a name for your program settings via the buttons favorite 20 store locations are available 5) Push OK to save the favorite for the Endomed 182 3) Push OK to confirm Page 35 of 52 EN109-1634750-40 IFU...
- Page 36 Loading and Deleting Favorites 1) Select “Favorites” in the 2) To load a program select a 3) To delete a Favorite press Home menu Favorite via the buttons the delete button (N) and select the Favorite to be The parameter screen will removed via the ...
-
Page 37: Application Information
Connection of electrodes See chapter 3 for description of numbered parts. Endomed 182 Type 1 Connect the DC reference [5] cable to connection [3] • Connect the blank earth electrode [4] to the DC reference cable [5] •... - Page 38 Endomed 182 Type 2 Connect the patient cables to connection 1 [10] and/or connection 2 [11] on the Current • Module [9] (red plug into red connection, black plug into black connection). Connect the electrodes to the patient cable. •...
- Page 39 25 mm – 30 mm • Foot switch The Endomed 182 Type 1 has an optional foot switch which can be connected to connection A [7] (see chapter 3). Place the footswitch so that it can be reached easily during treatment.
- Page 40 Endomed 182 Type 2 and 3: TENS / IFC Protocol for the safe application of TENS / IFC Check contraindications with patient • Test skin for normal sensation using blunt/sharp test • Device should be switched off and electric leads disconnected •...
- Page 41 It is important that electrodes remain in complete contact without edges lifting throughout the • treatment. The Endomed 182 output mode is Constant Current (CC). This will maintain the set current • amplitude, even when the impedance of the sponge pads increases during treatment caused by water evaporation.
- Page 42 Endomed 182 Type 3: Vacuum electrodes There is a choice of large, medium and small electrodes. The areas of the electrodes correspond to those of the 4 x 6 cm, 6 x 8 cm and 8 x 12 cm flexible rubber electrodes. The vacuum electrodes are...
- Page 43 Current density In the particular standard for Electrical Nerve and Muscle Stimulators, IEC 60601-2-10, it is recommended not to exceed a current density of 2 mA r.m.s. / cm², otherwise skin irritations or etching can occur. To find the maximum recommended current amplitude in mA for the Interferential current waveforms, multiply the electrode surface in cm²...
-
Page 44: Maintenance And Troubleshooting
12 Maintenance and troubleshooting Cleaning and Disinfection Clean the device and its accessories regularly to maintain hygienic safety. Before performing any user maintenance, switch off the device and disconnect the plug from the mains supply. Do not spray the cleaning agent directly on the glass panel. Do not use cleaning agents that contain strong alkalis, lye, acid, detergents with fluoride or detergents with ammonia. - Page 45 Vacuum The vacuum electrodes and sponges should be cleaned with lukewarm water. electrodes and In the case of persistent dirt, and for disinfection, a 70% alcohol solution may sponges be used. Sponges should be replaced regularly. It is recommended to keep sponges and a spare electrode in stock.
- Page 46 Error messages When the device is turned on, it will first execute a self-test. When an error is detected, both during the self-test and during normal operation, a pop-up screen will appear on the display. When the error is displayed, all outputs will be disabled. When this situation occurs, switch off the unit and disconnect all accessories.
- Page 47 The service engineer will decide whether the device is suitable for use according to the specifications. End of Life The Endomed 182 contains materials which can be recycled and/or are noxious for the environment. Please ensure that you are well informed of the local rules and regulations regarding the removal of equipment and accessories.
-
Page 48: Specifications
Frequency 50/60 Hz Max. power output 50 VA Dimensions (width x length x height) Endomed 182 Type 1 14.5 x 17 x 9 cm Endomed 182 Type 2 14 x 16 x 14 cm Endomed 182 Type 3 14 x 22.5 x 17.5 cm... - Page 49 Asymmetrical biphasic pulsed current (TENS) Pulse width 150 μs Frequency 1 – 200 Hz Modulation frequency 0 – 180 Hz Modulation program 1-1, 6-6, 1-30 seconds Burst frequency 1-2-4 Hz Technical modifications reserved Safety and performance standards Medical device classification IIa;...
- Page 50 Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm to any part of the Endomed 182, including cables specified by the manufacturer. Otherwise degradation of the performance of this equipment could result.
-
Page 51: Contact
14 Contact For assistance, please visit our website http://www.enraf-nonius.com The latest version (in electronic or printed format) of this Instructions for Use can be obtained free of charge from our website www.enraf-nonius.com or by contacting distributor or by calling the telephone number: +31-(0)10-2030600. - Page 52 Copyright: Enraf-Nonius B.V. Vareseweg 127 | 3047 AT | Rotterdam | The Netherlands Tel: +31 (0)10-20 30 600 | info@enraf-nonius.nl EN109-1634750-40...

















Need help?
Do you have a question about the Endomed 182 and is the answer not in the manual?
Questions and answers