Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for Zimmer Soleo Sono
- Page 1 Instructions for Use Soleoline Soleo Sono...
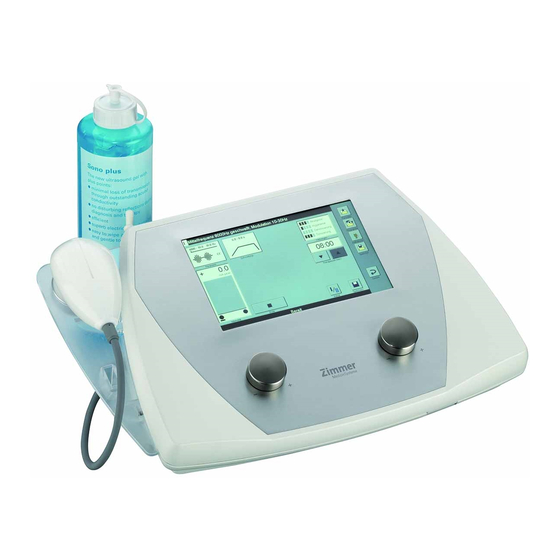
- Page 2 Illustrations Soleo Sono Front of the device Fig. 1 Device and 1 Intensity regulator channel I operating 2 Controller for frequency component elements 3 Display 4 Slot for SD card 5 Transducer 6 Holder for transducer (optional)
- Page 3 Illustrations Soleo Sono Display views / navigation menus Fig. 2 Display view 7 Status bar 8 Buttons on the screen 9 Navigation menus Fig. 3 Navigation menu (A) Back Moves one step back Description of the (B) Therapy Switches to therapy area...
- Page 4 11 On/off switch ports 12 Holder for mains fuse 13 Connection for power cable 16 Port for transducer 0.8 / 2.4 MHz 18 Identification plate Note: The ports (14*), (15*) and (17*) do not have any function for Soleo Sono.
- Page 5 Illustrations Soleo Sono Transducers Fig. 5 Transducer, large Transducer, small...
-
Page 6: Explanation Of Symbols
Explanation of symbols In the instructions for use this symbol indicates Danger. In the instructions for use this symbol indicates "Caution“ with regard to Caution possible damage of the device. Applied part type BF Follow instructions for use. Instructions for use Serial number Item number Manufacturer... -
Page 7: Table Of Contents
Content Illustrations Soleo Sono Front of the device Display views / navigation menus Rear of the device / Switches and ports Transducers Explanation of symbols Page Indications / Contraindications 1.1 Indications 1.2 Contraindications Side effects Application information 3.1 General 3.2 Ultrasound Warnings Soleoline –... - Page 8 Content Operation instructions 9.1 Ultrasound therapy 9.2 Displays / buttons 9.3 Water bath treatment 9.4 SD card 9.5 Protocols 9.6 Lists of favourites - Retrieve programmes, edit list Technical information 10.1 General 10.2 Ultrasound Cleaning, disinfection CE mark / manufacturer Scope of delivery, accessories Device combinations Safety and maintenance...
- Page 9 Content Valid for the device Soleo Sono (NG). These instructions for use are an integral part of the device. They must be stored with the device and kept accessible at all times for anyone authorised to operate this device. The instructions for use are valid as of October 2016.
-
Page 10: Indications / Contraindications
Indications / Contraindications 1.1 Indications Indications from • Vertebrogenic pain syndrome, e.g. cervical syndrome orthopaedics, • Ankylosing spondylitis (only during the inflammation-free interval) surgery, • Joint diseases traumatology, • Rheumatoid arthritis (if heat treatment is indicated) rheumatology • Osteoarthritis • Periarthropathy •... -
Page 11: Contraindications
Indications / Contraindications 1.2 Contraindications General • Unclear pain symptoms contraindications • Diseases for which heat should not be used, such as acute inflammatory diseases • Diseases in which mechanical influences are contraindicated, such as phlebothrombosis • Limited blood flow •... -
Page 12: Side Effects
Side effects Side effects of No side effects are known if used correctly. ultrasound therapy Page 3... -
Page 13: Application Information
Inspect the device before use. If there is any damage, it must not be used. Caution Only accessories provided by Zimmer MedizinSysteme GmbH must be used. Caution The device may cause malfunctions or may interfere with the operation of Caution equipment in the vicinity. -
Page 14: Ultrasound
Application information 3.2 Ultrasound Handle transducer gently; rough handling can alter its characteristics. Do not allow sharp or pointed objects to come into contact with the ultrasound transducer since the aluminium head can be easily scratched. The use of coupling agents other than the special SonoPlus ultrasound gel can damage the transducer. -
Page 15: Warnings
Warnings This device is intended to be used exclusively by medical professionals. The use of the device out of the settings or applications specified in the instructions for use may lead to hazard by the uncontrolled emission of ultrasound energy. The patient must not be left unattended during therapy. -
Page 16: Soleoline In Brief
Soleoline in brief What is Soleoline? An ultramodern, innovative product line featuring 3 different device variants. Soleo Sono An ultramodern, innovative ultrasound therapy device. Soleo SonoStim An ultramodern, innovative combination device for electro- and ultrasound therapy with the option of additionally using VacoS. -
Page 17: Intended Use
Intended use The Soleoline product line includes 3 different device variants: Soleo Sono Ultrasound therapy device for treatment with therapeutic ultrasound. Soleo SonoStim Combination device for therapeutic ultrasound therapy and electrotherapy with the option of additionally using VacoS. Soleo Galva Electrotherapy device with the option of additionally using VacoS. -
Page 18: Device Set-Up
Device set-up 7.1 Mounting the cables Note: The cables have coloured arrows to aid with orientation for proper connection with the device. Ultrasound therapy Connect the transducer to the provided port (16). Connect power Connect the power cable to the provided port (13) and connect the cable to cable the mains. -
Page 19: Settings
Settings 8.1 General Note: The following descriptions refer to the default factory settings. Note: Changes to the default settings can only be made from the start-up screen. Start-up screen After the device has been switched on and the self-test performed, the start- up screen opens. - Page 20 Settings 8.1 General The setting options are described below. The default settings are pre-programmed in the factory as shown on the screen. (1) Start-up settings Options to individually select the start-up settings. The selection is made directly in the corresponding line. (2) Language Language selection.
-
Page 21: Ultrasound Therapy
Settings 8.2 Ultrasound therapy Note: Activation (13) opens the screen "Configuration - Ultrasound". Ultrasound settings (1) Unit Option to adjust the intensity unit on the bar graph. The selection is made directly in the corresponding line. (2) Coupling The limit value of the coupling can be adjusted (50 to 95%). The adjustment can be made using the two arrow buttons. - Page 22 Settings 8.3 Maintenance Maintenance Activation (1) opens the maintenance menu. Software updates can be performed in the maintenance menu. You will receive current information on performing a software update if an update is planned. Enter the password "armin" to open the maintenance menu. All other points listed are not relevant for the user.
- Page 23 Activation (1) opens the programme screen. Here the desired programme is selected. Programmes Soleo Sono There are 9 different forms of therapy available in Soleo Sono: Select programme The desired ultrasound therapy programme is selected directly in the corresponding line.
- Page 24 Operation instructions 9.1 Ultrasound therapy Therapy screen After the ultrasound therapy programme has been selected, the therapy screen opens. Note: Before starting the treatment, check whether the data in the parameter window (here 5 cm²) match the transducer connected. Adjust intensity Adjust the intensity using the left intensity regulator (1).
- Page 25 Operation instructions 9.1 Ultrasound therapy Therapy start The therapy starts with (3). (3) switches to (4). The dose set is shown in the bar graph and the therapy time decreases every second. The coupling display is enabled. End of therapy After the therapy time has elapsed, an acoustic signal indicates the end of therapy and the clock is at 00:00.
-
Page 26: Displays / Buttons
Operation instructions 9.2 Displays / buttons Description of the display elements and buttons (1) Intensity The bar graph displays the currently set intensity. The intensity is adjusted using the left controller. (2) Start/Stop Activation (2) starts or stops the therapy, respectively. (3) Therapy time Displays the therapy time for the selected programme. -
Page 27: Water Bath Treatment
Operation instructions 9.3 Water bath treatment Note: If the ultrasound therapy is performed in a water bath, the temperature monitoring of the transducer must be modified prior to the therapy. Implementation Activation (1) opens the ultrasound parameter window. Activate water bath Through activation (2) and confirmation, the temperature monitoring of the button transducer is modified for therapy in a water bath. -
Page 28: Sd Card
Operation instructions 9.4 SD card SD card The user-defined settings as well as the list of indications are saved on the SD card. If the SD card is not inserted, the following message appears when the "Favourites" or "Indications" buttons are pressed: "No SD card found."... -
Page 29: Protocols
Operation instructions 9.5 Protocols Provides support when selecting a therapy and makes a therapy recommendation. Protocols (1) opens the menu with the therapy recommendations. Therapy selection The body region is selected by clicking on the white square. via body region Select clinical picture After selecting the desired body region, a window with various diseases opens in the shoulder area. - Page 30 Operation instructions 9.5 Protocols Therapy information After selecting the clinical picture, another window with detailed therapy information and a programme recommendation opens. Select therapy Activating (1) opens the therapy screen with the corresponding programme. programme Page 21...
-
Page 31: Lists Of Favourites - Retrieve Programmes, Edit List
Operation instructions 9.6 Favourites list Retrieve programmes / Edit list The parameters of the predefined programmes can be individually modified and saved. Save and name programme Activation (1) opens the keyboard to enter the programme name. The programme name can be either accepted or entered via the keyboard. Note: There are 120 storage locations available in each case. - Page 32 Operation instructions 9.6 Favourites list Retrieve programmes / Edit list The individually saved programmes are listed in the Favourites list. These can be retrieved here for therapy, edited (moved in the sequence and deleted). Select Favourites list Activation (1) opens the Favourites list. Retrieve programme The desired programme is selected directly in the corresponding line and opens the therapy screen.
- Page 33 Operation instructions 9.6 Favourites list Retrieve programmes / Edit list Edit Favourites Activation (1) moves the programme up. Activation (2) moves the programme down. Activation (3) deletes the programme. Activation (4) confirms the edit. Activation (3) triggers a confirmation prompt: Activation (5) deletes the programme.
-
Page 34: Technical Information
Technical information 10.1 General Mains voltage 100 - 240 V, 220 V / 50 Hz / 60 Hz 220 V / 60 Hz Mains fuse 2 x T2AL, 250 V, 5 x 20 mm Power consumption max. 60 VA Protection class Applied part Type BF Operating mode... -
Page 35: Ultrasound
Technical information 10.2 Ultrasound Transducers Frequency 0.8 MHz and 2.4 MHz Small transducer 1 cm² , ERA = 0.67 cm² at 0.8 MHz, 0.65 cm² at 2.4 MHz Maximum output 1.0 W at 0.8 MHz, 0.5 W at 2.4 MHz Intensity levels 0.1 to 1 W/cm²... -
Page 36: Cleaning Disinfection
Cleaning Disinfection - Before starting any maintenance and cleaning measures the device must always be switched off at the main switch and the mains cable must be disconnected. - Make sure that when cleaning and disinfecting the labelling of the device (such as warnings, labels of control devices, identification plate) is not damaged. -
Page 37: Ce Mark / Manufacturer
CE mark / Manufacturer The devices have a CE mark in accordance with the EC directive on medical devices 93/42/EEC. Manufacturer Zimmer MedizinSysteme GmbH Junkersstraße 9 D-89231 Neu-Ulm Tel. +49 731. 9761-0 Fax +49 731. 9761-118 www.zimmer.de Page 28... -
Page 38: Scope Of Delivery Accessories
Scope of delivery Accessories Scope of delivery Soleo Sono Item no. 5312-01 1 basic device (see below) 1 sliding frequency transducer 0.8 and 2.4 MHz, ø 28 mm 1 power cable 1 instructions for use Accessories Item no. 4200 Sliding frequency transducer 0.8 and 2.4 MHz, ø 28 mm 4220 Sliding frequency transducer 0.8 and 2.4 MHz, ø... -
Page 39: Device Combinations
Device combinations For Soleo Sono, no combination devices are provided by the manufacturer. Anyone who contrary to these specifications combines devices and thus operates a medical system does so at its own risk. Page 30... -
Page 40: Safety And Maintenance
Safety and maintenance Soleo Sono is manufactured according to the EN 60601-1 safety regulations. As the manufacturer, Zimmer MedizinSysteme can only consider itself to be responsible for safety and reliability if • the device is operated using a proper power outlet with earth contact and the electrical installation complies with DIN VDE 0100 part 710, •... -
Page 41: Functional Test
Functional test After being switched on, Soleo Sono performs a self-test which checks all internal components. If an error occurs, an error message will appear. In addition, an enhanced function test as described below can be performed. These tests should be performed monthly or if the proper functioning of the device is in doubt. -
Page 42: Safety Check Metrological Control
1 of the ordinance. A metrological control according to section 11 of the MPBetreibV (Medical Device Operator Ordinance) is also not required for Soleo Sono. The device is not listed in attachment 2 of the ordinance. -
Page 43: Error Messages Troubleshooting Disposal
The device may only be returned to the factory in its original packaging. For questions or problems with the device, please contact us at the address below: Main office Zimmer MedizinSysteme GmbH Junkersstraße 9 D-89231 Neu-Ulm Tel. +49 731. 9761-0 Fax +49 731. -
Page 44: Manufacturer's Emc Declaration
Portable and mobile HF communication devices (such as mobile phones, cell phones) can affect medical electrical devices. Soleo Sono may only be operated with the original parts indicated in the list of the scope of delivery and accessories. Operation of the device with other parts can lead to increased emissions or reduced interference immunity of the device! Guidelines and manufacturer's declaration –... - Page 45 Guidelines and manufacturer's declaration – Electromagnetic immunity The Soleo Sono device is intended to be operated in an electromagnetic environment as indicated below. The customer or user of the Soleo Sono device should ensure that it is used in such an environment.
- Page 46 Guidelines and manufacturer's declaration – Electromagnetic immunity The Soleo Sono device is intended to be operated in the electromagnetic environment as indicated below. The customer or user of the Soleo Sono device should ensure that it is used in such an environment.
- Page 47 If the measured field strength in the location in which the Soleo Sono device is used exceeds the above compliance level, the Soleo Sono device must be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the Soleo Sono.
- Page 48 Soleoline Instructions for Use Zimmer MedizinSysteme GmbH Junkersstraße 9 89231 Neu-Ulm, Germany Tel. +49 7 31. 97 61-291 Fax +49 7 31. 97 61-299 export@zimmer.de www.zimmer.de...













Need help?
Do you have a question about the Soleo Sono and is the answer not in the manual?
Questions and answers