Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for Acteon SATELEC Newtron Booster
- Page 1 User Manual Newtron Booster...
-
Page 3: Table Of Contents
Contents 1 Documentation 1.1 Associated documentation 1.2 Electronic documentation 2 Required information 2.1 Indication for use 2.2 Operating principle 2.3 Date of inclusion of EC marking 2.4 Latest document update 2.5 Repairing or modifying the device 2.6 Accessory usage conditions 3 Removal from packaging, installation, connections 3.1 Unpacking your medical device 3.2 Positioning the medical device... - Page 4 5.2 Cleaning and disinfecting accessories 6 Monitoring and maintenance of the medical device 7 Maintenance 7.1 Replacing the water filter 7.2 Identifying incorrect operation 7.2.1 No operation 7.2.2 No spray 7.2.3 The power is not as expected 7.2.4 Ultrasounds not working 7.2.5 Water leakage 8 Technical specifications for the medical device 8.1 Identification...
- Page 5 Foreword The medical device SATELEC ® that you are about to install and use in your practice is a medical device designed for professional use. It comprises the chosen tool with which you will provide treatment within the context of your work. To ensure optimum safety for yourself and your patients, comfort in your daily practice and to benefit fully from the technology of your medical device, please read the documentation provided carefully.
-
Page 7: Documentation
1 Documentation This document contains the following information: indications for use; description of the medical device; installation of the medical device; use of the medical device; preparation for cleaning and disinfection of the medical device; monitoring and general maintenance of the medical device; maintenance to be performed by the user. - Page 8 - User Manual • Newtron ® Booster • J60119 • V3 • (13) • 11/2013 • NBABUS030C Page...
-
Page 9: Required Information
2 Required information 2.1 Indication for use This medical device is used in association with a dental ultrasound handpiece to which an ultrasound instrument is attached. It is intended for prophylaxis, including scaling, for periodontics, for endodontics, and for preservation and restoration dentistry, including prosthesis. 2.2 Operating principle An electrical signal emitted by the medical device is supplied to the dental ultrasonic handpiece. - Page 10 - User Manual • Newtron ® Booster • J60119 • V3 • (13) • 11/2013 • NBABUS030C Page...
-
Page 11: Removal From Packaging, Installation, Connections
3 Removal from packaging, installation, connections 3.1 Unpacking your medical device When you receive your medical device, check for any damage that may have occurred during transportation. If you have received this medical device by mistake, please contact the supplier to arrange for it to be collected. -
Page 12: Connecting The Medical Device To The Electrical Network
The water supply system must comply with the quality criteria compatible with the practice of dental treatments. 3.5 Connecting the medical device to the electrical network Set the medical device to OFF position O and check that the mains voltage is compatible with that indicated on the medical device or its mains adapter. -
Page 13: Description Of The Medical Device
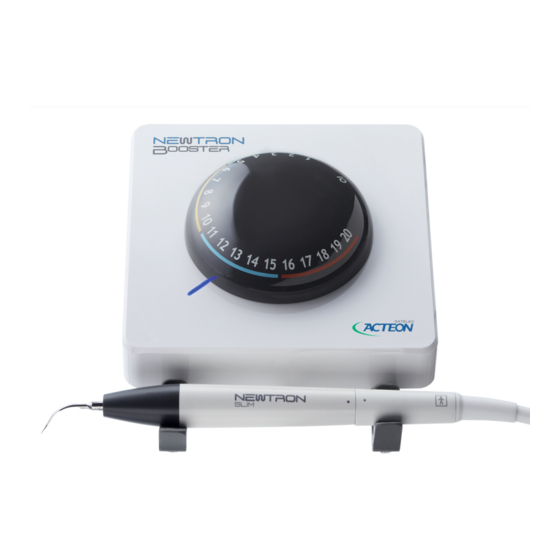
4 Description of the medical device 4.1 Control unit The control unit incorporates technology Newtron ® patented by SATELEC ® . The patented technology Newtron ® controls the tips by Cruise Control ® , an automatic system for setting the frequency and power in real time. -
Page 14: Handpiece Support
Lubricate the irrigation system seal located behind the with dental instrument lubricant such as ClearView™ Dental Handpiece Lubricant manufactured by Dental Air Solutions to extend its effectiveness and prevent leaks. Connect the to the sleeve, by aligning the indexing points and by avoiding rotation movement. Install the on the support. -
Page 15: Activating Ultrasounds Using The Pedal
4.17 Activating ultrasounds using the pedal To activate the ultrasounds on your medical device, press the control pedal. 4.18 Electrical Adapter The medical device device is designed to be connected to a mains adapter, which is considered as being an integral part of the medical device device. - Page 16 - User Manual • Newtron ® Booster • J60119 • V3 • (13) • 11/2013 • NBABUS030C Page...
-
Page 17: Cleaning, Disinfecting And Sterilizing
5 Cleaning, disinfecting and sterilizing The instructions relating to accessory cleaning, disinfection and sterilization protocols provided by SATELEC ® have been approved for each medical device and accessory. The applicable guides are listed in chapter Associated documentation page 5 They can be downloaded at the following address: www.satelec.com/documents In all cases, the local regulations in force relating to the accessory cleaning, disinfection and sterilization protocols take precedence over the information provided by SATELEC ®... - Page 18 To prepare for cleaning, remove the various parts of Newtron ® Booster as shown here. 5.2 Cleaning and disinfecting accessories Refer to the accessory cleaning, disinfection and sterilization protocols listed in the chapter Associated documentation page 5. The diamond coated inserts cannot be reprocessed since they cannot be cleaned properly. Bone and soil residues might remain adhered to the diamon coating even after cleaning and sterilization and enter into the oral cavity of another patient.
- Page 19 6 Monitoring and maintenance of the medical device Before and after each use, check that the device and its accessories are not faulty in any way. This is necessary to detect any isolation fault or damage. If necessary, replace damaged parts. Monitor the cleanliness of the air inlets on the control unit to prevent any heating.
- Page 20 - User Manual • Newtron ® Booster • J60119 • V3 • (13) • 11/2013 • NBABUS030C Page...
- Page 21 7 Maintenance The only preventive maintenance the medical device requires is: checking of accessories; everyday cleaning, disinfection and sterilisation procedures; cleaning ; replacement of the water filter cartridge. 7.1 Replacing the water filter The water filter must be cleaned regularly and must be replaced every 6 months. Proceed as follows: shut off the water supply;...
- Page 22 Possible causes Solutions Dental cabinet water inlet in shut-off position Open the water inlet Flow configuration button on minimum Adjust the flow configuration button Faulty water pipe connection Check the water inlet Low water pressure Check the water system pressure Blocked filter Clean or change the filter ®...
- Page 23 8 Technical specifications for the medical device 8.1 Identification ® Manufacturer SATELEC ® B Name of the medical device EWTRON OOSTER 8.2 Mains Adapter Manufacturer CINCON ELECTRONICS CO.LTD Model TR60M36 Supply voltage 100 VAC - 240VAC Power supply frequency 47Hz - 63Hz Power consumption 60 W Output voltage...
- Page 24 8.5 Length of cords Scaler handpiece cord (in mm) 2 040 Control pedal cord (in mm) 2 000 8.6 Irrigation Water pressure at inlet 1 to 5 bars Maximum water output flow at the end of the handpiece 80 ml/min to 100 ml/min at 5 input bars 8.7 Control pedal Width (in mm) Height (in mm)
- Page 25 9 Regulations and standards 9.1 Official Texts This medical device complies with the essential requirements of European Directive 93/42/EEC. This equipment is designed and developed in compliance with Electrical Safety standard IEC60601-1 in force. It was designed and manufactured in accordance with an EN ISO 13485-certified quality assurance system. 9.2 Medical class of the device This medical device is a class IIa device according to European Directive 93/42/EEC.
- Page 26 9.3 Standardised Symbols Symbols Meaning Refer to the accompanying documentation Consult the User Manual Accompanying documentation in electronic format BF type Class 2 Alternating voltage Sterilisation at 132°C in an autoclave Washer disinfector for thermal disinfection EC marking Do not dispose of as household waste Year of manufacture YYYY Control pedal...
- Page 27 9.4 Manufacturer identification SATELEC A Company of ACTEON Group 17, avenue Gustave Eiffel BP 30216 33708 MERIGNAC cedex FRANCE Tel. +33 (0) 556.34.06.07 Fax. +33 (0) 556.34.92.92 E.mail: satelec@acteongroup.com. www.acteongroup.com User Manual • Newtron ® Booster • J60119 • V3 • (13) • 11/2013 • NBABUS030C - Page...
- Page 28 ACTEON INDIA B-94, GIDC Electronic Estate - Sector 25 – SPAIN GANDHINAGAR 382028 Gujarat - INDIA ACTEON MEDICO-DENTAL IBERICA, S.A.U. Tel. +91 79 2328 7473 Avda Principal n°11 H Fax. +91 79 2328 7480 Poligono Industrial Can Clapers e-mail: info@in.acteongroup.com...
- Page 29 : sandy.junior@au.acteongroup.com TAIWAN ACTEON TAIWAN 14F-1, N°433, Jinping Rd. Jhonghe Dist., New Taipei City 23563 TAIWAN (R.O.C) Tel. + 886 926 704 505 e-mail : tina.chu@tw.acteongroup.com - User Manual • Newtron ® Booster • J60119 • V3 • (13) • 11/2013 • NBABEN030CNBABUS030C...
- Page 30 When your medical device has reached the end of its service life, contact your nearest dental equipment dealer, or ACTEON GROUP head office or one of the company branches to find out how to proceed. The relevant contact details are given in the chapter Branch addresses page 26.
- Page 31 Index: – After installation mains adapter 10 Index fault 17 Filter 20 first inclusion of EC marking 7 first use 13 fuse 19 After installation 13 air circulation 12 air inlets 12, 17 Altitude 22 Amplitude 22 gas-filled atmosphere 22 approved dealers 7 general instructions relating to the comprehensive attachments 9...
- Page 32 Index: – Mains switch Wrenches Mains switch 19 Manufacturer 21 Medical class 23 ultrasonic vibrations 7 ultrasound power 11 update 7 user instructions 5 non-use 13 User Manual 5 pedal 12 Vibration frequency 22 periodontics 7 power configuration button 10 power settings 11 preservation and restoration dentistry 7 Pressure 22...
- Page 33 Ref : J60119 • V3 • (13) • 11/2013 • NBABUS030C A Company ofACTEON Group• 17 av.GustaveEiffel • BP30216 • 33708 MERIGNAC cedex • France Tel.+33 (0)556 34 06 07 • Fax.+33 (0)556 34 92 92 E-mail:satelec@acteongroup.com • www.acteongroup.com...
















Need help?
Do you have a question about the SATELEC Newtron Booster and is the answer not in the manual?
Questions and answers