Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for Hillrom MetaNeb
- Page 1 The MetaNeb® System Instructions for Use Product No. PMN4...
- Page 3 This manual (174432) was originally released and supplied in English. For a list of available translations, contact Hill-Rom Technical Support. The MetaNeb® System Instructions for Use (174432 REV 10)
- Page 4 Hill-Rom® is a registered trademark of Hill-Rom Services, Inc. Hillrom is a trademark of Hill-Rom Services, Inc. MetaNeb® and MetaTherapy® are registered trademarks of Comedica, Inc. Replace this manual (174432) if it is damaged and/or can not be read. For product support or to order additional copies of this manual (174432), contact your distributor, local Hill-Rom representative, or go to www.hill-rom.com.
-
Page 5: Table Of Contents
Autocephalad Flow ........4 The MetaNeb® System and Bronchial Hygiene... . . 5 Symbols . - Page 6 Re-Evaluation ..........24 The MetaNeb® System In-Line with Ventilator Protocol ... 24 Frequency .
-
Page 7: Indications
Indications INDICATIONS NDICATIONS FOR The MetaNeb® System is indicated for mobilization of secretions, lung expansion therapy, the treatment and prevention of pulmonary atelectasis, and also has the ability to provide supplemental oxygen when used with compressed oxygen. ATIENT OPULATION •... -
Page 8: Precautions
• Make sure you connect the nebulizer to the nebulizer tubing only. • Only persons trained to use The MetaNeb® System and ventilators should perform therapy on ventilated patients. • Unlock the brake casters during transport. Lock the brake casters during therapy or when not being transported. -
Page 9: Introduction
The MetaNeb® System supplies a therapy that enhances secretion removal and helps prevent or resolve patchy atelectasis. ESCRIPTION The MetaNeb® System is a therapeutic device that uses a systematic approach to enhance normal mucus clearance and resolve or prevent patchy atelectasis. -
Page 10: Theory Of Operation
Essentially, autocephalad flow is comparable to a cough, except that lower gas velocities are generally present. To further understand, it is helpful to examine normal tidal The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 11: The Metaneb® System And Bronchial Hygiene
The MetaNeb® System and Bronchial Hygiene The MetaNeb® System supplies aerosol, CHFO, and CPEP therapy modes. CHFO is a pneumatic form of chest physiotherapy that uses a systematic approach to enhance normal mucus clearance and resolve patchy atelectasis. -
Page 12: Symbols
To ignore a warning could cause patient or user injury. – A CAUTION identifies special procedures or precautions that persons must obey to help prevent equipment damage. The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 13: Product Symbols
Aerosol mode—CHFO and CPEP are not avail- able during this mode. Higher/Lower—used with CHFO. Flow—used with CPEP. Fire Hazard—keep away from ignition sources. This product is not manufactured with natural rubber latex. Manufacturer and date of manufacture The MetaNeb® System Instructions for Use (174432 REV 10) - Page 14 The CE mark was first applied in 2014. The mark applies only to a controller (Part number 186071). The MetaNeb® System Instructions for Use (174432 REV 10)
-
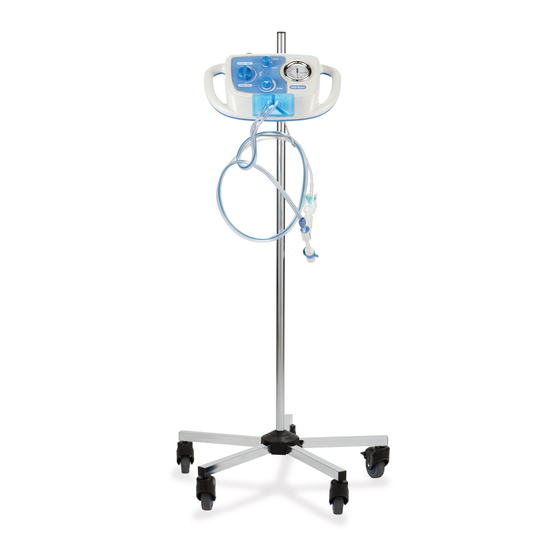
Page 15: Features
Features FEATURES ONTROLLER Item Description Item Description Pressure manometer CPEP flow adjuster Higher/lower switch Master ON/OFF switch Mode selector Oxygen gas connector Circuit tri-connector port H Mount bracket The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 16: Circuit
Item Description Mouthpiece Orifice indicators Selector ring Adapter, 22 mm x 15 mm Handset Adapter, 22 mm x 22 mm Circuit tri-connector/ Occlusion ring bio-filter Tubing Silicon adapter Nebulizer Nebulizer tubing The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 17: Assemble The Controller System
If a two parts pole construction is used, make sure the two parts are fully tightened to each other and there is no gap at the joint. Installation of 183808 and 207384 Stands The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 18: Installation Of M08183 Stand
Assemble the Controller System Installation of M08183 Stand The MetaNeb® System Instructions for Use (174432 REV 10) - Page 19 Assemble the Controller System The MetaNeb® System Instructions for Use (174432 REV 10)
-
Page 20: Connect The Oxygen Hose
45° angle and gently push it in and twist it to the correct orientation. CAUTION: Caution—Use only with the supplied nebulizer. Remove the nebulizer from the package. Assemble the nebulizer and add the prescribed medication. See “Add Medication to the Nebulizer” on page 19. The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 21: Other Configurations
Mask NOTE: It may be difficult to connect the adapter to the facemask. If needed, apply more pressure when you are connecting the two parts. Tracheostomy Flex tube adapter not included The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 22: In-Line With Ventilator Circuit
Specifications of the bacterial filter that can be used with the MetaNeb® System: • Bacterial Filtration efficiency: > or equal to 99% •... - Page 23 Assemble the Circuit You can use a bacterial filter in-line with the MetaNeb® System using one of the configurations: Configuration 1: Using a Bacterial Filter with the MetaNeb® System NOTE: This configuration is not for use with a nebulizer. However, you may connect a nebulizer if you do not add any medication or saline.
-
Page 24: Add Medication To The Nebulizer
Install and tighten the container cap on to the container. Make sure the green cone (2) in the container stays in position. The MetaNeb® System Instructions for Use (174432 REV 10) - Page 25 20 and not more than 30 cm H NOTE: If the device is not within the parameters specified above, contact Hill- Rom Technical Support to examine, troubleshoot, and repair the unit (if needed). The MetaNeb® System Instructions for Use (174432 REV 10)
-
Page 26: Pre-Use Check
20 and not more than 45 cm NOTE: If the device is not within the parameters specified above, contact Hill- Rom Technical Support to examine, troubleshoot, and repair the unit (if needed). The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 27: Pre-Use Check For In-Line Use With A Ventilator
The patient should be assessed per institutional guidelines. Fill the nebulizer with the prescribed medications, if applicable. See “Using a Bacterial Filter In-Line with the MetaNeb® System” on page Set the mode to CPEP. Turn the CPEP flow dial all the way clockwise to the lowest position. -
Page 28: Metatherapy® Treatment Protocol
25. Disconnect the circuit, and store the unit for future use. NOTE: When you store the circuit, keep the tubing attached to the bottom of the nebulizer cup and disassemble the top of the nebulizer from the handset. The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 29: Assessment Of Outcome
Warning—Only persons trained to use The MetaNeb® System and ventilators should perform therapy on ventilated patients. REQUENCY In-line use of The MetaNeb® System with a ventilator ranges in frequency from four (4) to eight (8) times daily as determined by the patient's response to the therapy. -
Page 30: The Metaneb® System In-Line With Ventilator Protocol
Fill the nebulizer with the prescribed medications as applicable. See “Using a Bacterial Filter In-Line with the MetaNeb® System” on page Connect The MetaNeb® System to an approved 50 psi oxygen source. 10. Set the mode to CHFO and select Higher. -
Page 31: Assessment Of Outcome
The post therapy chest exam shows an absence of retained secretions and atelectasis. • Breath sounds have become clear or have improved. VALUATION Patients should be evaluated every 24 hours while on The MetaNeb® System to make sure that an acute change has not occurred. MOVE THE STAND NOTES: •... -
Page 32: Cleaning
Warning—Do not use phenolic, alcohol, quaternary ammonium chloride, or bleach solutions to clean the patient circuit. Use these solutions to clean the controller only. The Metaneb® Controller has been tested for compatibility with these cleaning and disinfecting solutions: Chemical Class... -
Page 33: Cleaning The Controller And Stand
Let the handset, nebulizer cup, mouthpiece, and adapters air dry or dry with a lint free cloth or paper towel. 10. Wipe down the outside of the circuit tubing with an approved alcohol based cleaner. The MetaNeb® System Instructions for Use (174432 REV 10) - Page 34 MetaNeb® System” on page 16. • Do the “Pre-Use Check” on page 20. • For units used with an in-line ventilator, do the “Pre-Use Check For In- Line Use with a Ventilator” on page 21. The MetaNeb® System Instructions for Use (174432 REV 10)
-
Page 35: Troubleshooting And Maintenance
Make sure the Mode selector switch and the On/Off switch are in the correct position. Unit DISS connection. Make sure the unit is connected to an approved 50 psi oxygen source. The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 36: Maintenance
For disposal of the controller, return it to Hill-Rom or your Hill-Rom distributor. HIPPING AND ACKAGING When The MetaNeb® System controller is shipped for repair, follow these shipping and packaging instructions: Request and get a return goods authorization (RGA) number from Hill-Rom. -
Page 37: Specifications
Base width 19" (48.3 cm) Rolling diameter 22" (55.9 cm) Oxygen Supply Hose (USA only; not applicable for other countries) Color Green Length 10' (304 cm) Fittings Standard DISS on each end The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 38: Controller Dimensions
Single and 5 pack options are available (see “Circuit Part Numbers” on page 43). b. The supply pressure is regulated to 50 psi (345 kPa) if a higher pressure source is connected. ONTROLLER IMENSIONS The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 39: Stand Dimensions (183808 And 207384)
Specifications (183808 and 207384) TAND IMENSIONS The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 40: Stand Dimensions (M08183)
Specifications (M08183) TAND IMENSIONS The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 41: Classification And Standards
Technical and Quality Assurance ISO 13485 EN13544-1 FDA Medical Device Equipment Class II Classification Classification According to Medical Device Directive 93/42/EEC 1. These conditions and values apply to a fully-assembled MetaNeb® System. The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 42: Technical Specification For Performance-Breathing Simulator
(345 to 379 kPa) and the Breathing Simulator settings shown here: Breathing Simulator Settings Lung Resistance 20 cm H O/l/s Lung Compliance 50 mL/cm H Breath Rate 20 bpm Effort Amplitude 25 cm H Target Volume 500 mL The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 43: Chfo Pulses-Airway Pressure Reading
240 ± 60 bpm (300 bpm max) CHFO Low Rate 70% of CHFO High Rate (100 bpm min) Peak Pressure 30 cm H Oscillating Amplitude 5 cm H O minimum CPEP—A IRWAY RESSURE EADING The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 44: Hi Pressure Chfo Pulses-Airway Pressure Reading
240 ± 60 bpm (300 bpm max) CHFO Low Rate 70% of CHFO High Rate (100 bpm min) Peak Pressure 40 cm H Oscillating Amplitude 5 cm H O minimum CPEP—A RESSURE IRWAY RESSURE EADING The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 45: Bench Performance Checks-Breathing Simulator
For these checks, we used a compressed gas supply of 50 to 55 psi (345 to 379 kPa). The breathing simulator was configured with different compliance and resistance values to represent different degrees of lung health. The MetaNeb® System Instructions for Use (174432 REV 10) - Page 46 15 bpm Effort Amplitude 15 cm H Effort Slope Effort% Inhale Effort Offset 0 cm H Target Volume 650 mL Flow Conversion Factor Time between Samples 30.1 milliseconds Number of Samples The MetaNeb® System Instructions for Use (174432 REV 10)
- Page 47 15 bpm Effort Amplitude 15 cm H Effort Slope Effort % Inhale Effort Offset 0 cm H Target Volume 650 mL Flow Conversion Factor Time between Samples 30.1 milliseconds Number of Samples The MetaNeb® System Instructions for Use (174432 REV 10)
- Page 48 15 bpm Effort Amplitude 15 cm H Effort Slope Effort % Inhale Effort Offset 0 cm H Target Volume 650 mL Flow Conversion Factor Time between Samples 30.1 milliseconds Number of Samples The MetaNeb® System Instructions for Use (174432 REV 10)
-
Page 49: Nebulizer Performance
Representative Particle Size Distribution per EN13544-1 CIRCUIT PART NUMBERS Part Number Description C20000N Circuit, SPU With Nebulizer, Single Kit PC20005N Circuit, SPU With Nebulizer, 5 Piece Kit The MetaNeb® System Instructions for Use (174432 REV 10) -
Page 50: Particle Specifications
<1.0 Microns - ug 34.7-52 1032.1-1489.1 NOTE: Coarse particles (due to oro-pharyngeal deposition) and ultra-fine particles (due to exhalation) are not likely to deposit in the patient’s airway and provide limited clinical benefit. The MetaNeb® System Instructions for Use (174432 REV 10) - Page 51 <1.0 Microns - ug 35.2-58.5 720.5-1153.5 NOTE: Coarse particles (due to oro-pharyngeal deposition) and ultra-fine particles (due to exhalation) are not likely to deposit in the patient’s airway and provide limited clinical benefit. The MetaNeb® System Instructions for Use (174432 REV 10)
- Page 52 <1.0 Microns - ug 30-47.3 307.4-673.4 NOTE: Coarse particles (due to oro-pharyngeal deposition) and ultra-fine particles (due to exhalation) are not likely to deposit in the patient’s airway and provide limited clinical benefit. The MetaNeb® System Instructions for Use (174432 REV 10)
















Need help?
Do you have a question about the MetaNeb and is the answer not in the manual?
Questions and answers