Zimmer enPuls Instructions For Use Manual
Hide thumbs
Also See for enPuls:
- Instructions for use manual (30 pages) ,
- User manual (46 pages) ,
- Operating instructions manual (50 pages)
Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Zimmer enPuls
- Page 1 Instructions for Use enPuls Version 2.0...
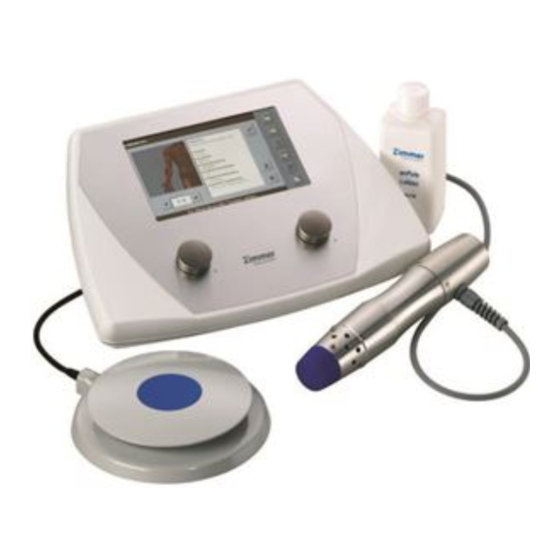
- Page 3 Illustrations Front of the device Fig. 1 Selection and 1 Control unit operating elements 2 Pulse energy controller 3 Display 4 Frequency controller 5 Slot for SD card Handpiece 6 Handpiece with applicator head 25 mm 7 Vents, front 8 Vents with fan, rear Foot switch 9 Foot switch...
-
Page 4: Illustrations
Illustrations Rear of the device Fig. 2 Switches / ports 10 Mains switch 11 Mains fuse 12 Port for power cable 13 Port for foot switch 14 Port for handpiece 15 Serial no./identification plate... -
Page 5: Screens / Display
Illustrations Screens / display Fig. 3 Display/ 16 Buttons on the screen therapy screen 17 Status line 18 Navigation menu Fig. 4 Navigation menu (A) Back Moves one step back Description of the (B) Therapy Switches to the therapy screen functions (C) Protocols Switches to Protocols... -
Page 6: Applicator Heads And Accessories
Illustrations Applicator heads and accessories Fig. 5 Applicator heads 19 Applicator head, 25 mm 20 Applicator head, 15 mm 21 Applicator head, 6 mm Accessories 22 Silicone protection cap... -
Page 7: Explanation Of Symbols
Explanation of symbols In the instructions for use this symbol indicates “Danger”. In the instructions for use this symbol indicates "Caution" with regard to Caution possible damage of the device. Connection port for handpiece Connection port for foot switch Instructions for use Follow instructions for use. -
Page 8: Table Of Contents
Applicator heads and accessories Explanation of symbols Page Indications / Contraindications Side effects Application information Warnings enPuls version 2.0 – in brief System set-up Default settings Operation instructions 8.1 Device description 8.2 Information on operation 8.3 Performing the treatment 8.4 Displays and buttons 8.5 SD card... - Page 9 Manufacturer's EMC declaration Error! Bookm Valid for the device enPuls version 2.0 (NG). These instructions for use are an integral part of the device. They must be stored with the device and kept accessible at all times for anyone authorised to operate this device.
-
Page 10: Indications / Contraindications
Indications / Contraindications Indications • Radial and ulnar epicondylitis • Calcifying tendonitis / shoulder problems • Condition post blunt muscle injuries • Chronic patellar tendon syndrome • Patellar tendonitis • Chronic tendinopathy of the Achilles tendon • Plantar fasciitis • Heel spurs •... -
Page 11: Side Effects
Side effects Side Effects Treatments with enPuls version 2.0 may occasionally cause irritation, petechiae, haematoma, swelling, or pain. Page 2... -
Page 12: Application Information
X-ray or diathermy equipment) may interfere with the operation of the device. Please keep a safe distance of several meters. enPuls version 2.0 is not suitable for use in areas with an explosive, flammable or combustive environment. During use, the device is to be located in a position allowing direct access to the device´s central mains supply so that it can be disconnected from the mains... -
Page 13: Warnings
Warnings In exceptional circumstances the treatment time is limited to 4 minutes followed by a break of at least 15 minutes. The handpiece can overheat if the treatment time is exceeded. Treatment instructions regarding treatment location, duration and intensity require medical knowledge and may only be given by authorised physicians, therapists and medical paraprofessionals. -
Page 14: Enpuls Version 2.0 - In Brief
It generates shock waves by means of an ergonomic handpiece and emits enPuls version 2.0 shock waves through special applicators. The enPuls version 2.0 can penetrate human tissue for up to approximately 35 mm. How are shock waves A coil generates an electromagnetic field in the rear of the handpiece. -
Page 15: System Set-Up
Device set-up Note: Remove the enPuls version 2.0 from the transport bag prior to starting up the system. Operation in the case is not intended. Ensure that enPuls version 2.0 is placed on a stable surface. Note: Make sure that the power switch of the device is set to “0”. -
Page 16: Default Settings
Default settings Note: Changes to the default settings can only be made from the start-up screen. Start-up screen After the device has been switched on and the self-test performed, the start-up screen opens. Note: Activating the "therapy" button (1) switches immediately to the therapy screen. Configuration menu Factory settings can be changed and individually adjusted in the configuration menu. - Page 17 Default settings (1) Start settings Options to individually select the start-up settings. The selection is made directly in the corresponding line. (2) Language Language selection. The selection is made directly in the corresponding line. (3) Welcome Activating the “Welcome text” field opens a window with an alphabetical keyboard to enter an individual welcome text in the start-up screen.
-
Page 18: Operation Instructions
Zimmer MedizinSysteme GmbH guarantees unrestricted use at a rate of at least two million shocks per shock wave generator. In some cases, depending on power and frequency, well over 2 million shocks can be emitted. - Page 19 In addition to temperature monitoring, the enPuls version 2.0 regulates the temperature using a temperature sensor in the handpiece. The fan in the handpiece is started when activated by the foot switch and automatically stops when it reaches a certain temperature.
-
Page 20: Information On Operation
While shock wave application is activated, you can work either with the handpiece stationary on one point, or dynamically across an area. We recommend using the enPuls lotion supplied to reduce friction on the skin. The weight of the handpiece means that it is usually not necessary to press down firmly on the treatment area / point. -
Page 21: Performing The Treatment
Set pulse energy Use the left-hand controller to set pulse energy. Note: enPuls Version 2.0 offers two pulse emission options. Pulse emission with pre-set number of pulses For pulse emission with a pre-set number of pulses, treatment is terminated by the device after the pre-set number of pulses has been emitted. -
Page 22: Displays And Buttons
Operation instructions 8.4 Displays and buttons Description of the display elements and buttons (1) Pulse energy Displays the set pulse energy. During active therapy, the bar graph is filled in. Pulse energy can be set before and during pulse emission. Pulse energy can be set in 10 mJ increments to between 60 and 185 mJ. - Page 23 Operation instructions 8.4 Displays and buttons (9) Reset For ascending counting direction, this resets to 0; for descending counting direction, this resets to the pre-set number of pulses. (10) Information Activating the button returns to the therapy and treatment information. (11) Status line Displays the name of the currently selected program.
-
Page 24: Sd Card
Operation instructions 8.5 SD card SD card The user-defined settings as well as the list of indications are saved on the SD card. If the SD card is not inserted, the following message appears when the "Favourites" buttons are pressed: "No SD card found."... -
Page 25: Protocols
Operation instructions 8.6 Protocols The “Protocols” menu is used for support during therapy selection. Protocols Activating the “Protocols” button opens the menu with the therapy recommendations. Note: The “Protocols” menu offers two ways of selecting the desired therapy: - via the body regions - via the list Therapy selection The body region is selected by clicking on the black square. - Page 26 Operation instructions 8.6 Protocols Select differentiated The differentiated condition of the clinical picture is selected directly in the condition of the corresponding line. clinical picture Therapy information After selecting the differentiated condition of the clinical picture, another window with detailed therapy and treatment information opens. Activating button (2) gives access to additional therapy and treatment information.
-
Page 27: Favourites List - Retrieve Programs, Edit List
Operation instructions 8.7 Favourites list Retrieve programs, Edit list The parameters of the predefined programs can be individually modified and saved. Save and name Activating the “Save” button opens the field to enter the program name. program The program name is entered via the keyboard. Note: There are 120 storage locations available. - Page 28 Operation instructions 8.7 Favourites list Retrieve programs, Edit list The individually saved programs are listed in the Favourites list. These can be 1. retrieved here for therapy, 2. edited (moved in the sequence and deleted). Select Favourites list Activating the “Favourites” button opens the favourites list. Retrieve program The desired program is selected directly in the corresponding line Edit Favourites list...
- Page 29 Operation instructions 8.7 Favourites list Retrieve programs, Edit list Edit Favourites Activating the button (1) leads back to the start-up screen. Activating the button (2) moves the program up. Activating the button (3) moves the program down. Activating the button (4) deletes the program. Activating button (5) confirms the edit.
-
Page 30: Technical Information
322 mm x 235 mm x 130 mm Weight 2.7 kg IP class Device IPX0 Foot switch IPX5 Handpiece IPX0 Handpiece enPuls 2.2 Dimensions Length 230 mm, diameter 50 mm Weight 850 g Length of service 2,000,000 shocks (at least) -
Page 31: Cleaning/Disinfection
Cleaning Disinfection – Before starting any maintenance and cleaning measures the device must always be switched off at the main switch and the mains cable must be disconnected. – Make sure that when cleaning and disinfecting the labelling of the device (such as warnings, labels of control devices, identification plate) is not damaged. - Page 32 Cleaning Disinfection Tools: Disposable wipes (cellulose, paper) Alcohol-free plastic cleaner (e.g. cleaner for medical devices) Remove the silicone protection cap from the applicator head prior to cleaning. Then follow the procedure described under "Housing / foot switch". Disinfection (only manually): Tools: Disposable wipes (cellulose, paper) ...
- Page 33 Cleaning Disinfection Cleaning/disinfection, mechanical: Preparation: Visible contamination must be removed manually prior to cleaning/disinfection. Proceed as indicated above. Procedure: Carry out mechanical cleaning and disinfection using the following parameters: Cleaning agent: neodisher® MediClean forte (manufacturer: Dr. Weigert) Cleaning: 10 minutes at 55°C ...
- Page 34 CE mark / manufacturer The device has a CE mark in accordance with the EC directive on medical devices 93/42/EEC. Manufacturer Zimmer MedizinSysteme GmbH Junkersstraße 9 89231 Neu-Ulm, Germany Tel. +49 731. 9761-291 Fax +49 731. 9761-299 www.zimmer.de Page 25...
- Page 35 Scope of delivery and accessories Scope of delivery Item no. 5418-01 enPuls version 2.0 control unit 5413 Handpiece version 2.2 93133521 Applicator head, 6 mm 93133511 Applicator head, 15 mm 93133502 Applicator head, 25 mm 65135110 10 Silicone protection caps...
- Page 36 Device combinations For enPuls version 2.0 no combination devices are provided by the manufacturer. Anyone who combines devices against these guidelines and thus creates a medical system does so under his/her own responsibility. Page 27...
- Page 37 Safety and maintenance 14.1 Safety enPuls version 2.0 is manufactured according to the DIN EN 60601-1 safety regulations. As the manufacturer, Zimmer MedizinSysteme can only consider itself to be responsible for safety and reliability if • the device is operated using a proper power outlet with earth contact and the electrical installation complies with DIN VDE 0100 part 710, •...
- Page 38 Before starting any cleaning and maintenance measures the device must always be switched off at the main switch and the mains cable must be disconnected. Zimmer guarantees 150,000 shocks per applicator head. It is recommended that the applicator head be replaced after this number of shocks has been reached.
- Page 39 Functional test After being switched on, enPuls version 2.0 performs a self-test which checks all internal components. If an error occurs, an error message will appear. In addition, an enhanced functional test as described below can be performed. This test should be performed monthly or if the proper functioning of the device is in doubt.
- Page 40 Legal notice The enPuls version 2.0 NG device is not listed in annex 1 of the MPBetreibV (German Medical Devices Operation Ordinance). The device is not listed in annex 2 of the MPBetreibV (German Medical Devices Operation Ordinance). In Germany, the German Social Accident Insurance (DGUV) (Regulation 3 –...
- Page 41 Error messages Troubleshooting Disposal Handpiece loss of The message "Ready" appears in the status bar but no pulse is triggered function although the foot switch has been activated Possible cause 1 Handpiece / foot switch not properly connected or defective. Remedy for cause 1 Check whether the foot switch and handpiece are correctly connected.
- Page 42 Error messages Troubleshooting Disposal Applicator not found The message “No applicator found” appears in the status bar. Possible cause Handpiece not (or not correctly) connected. Remedy for cause Check whether the handpiece is correctly connected. The connector must be fully engaged. Device malfunction No response to the main switch / display remains dark Possible cause 1...
- Page 43 In the case of other malfunctions, switch the device off and then on again after a 5-second delay. If the error is still present, please inform customer service via the main office in Neu-Ulm. Main office Zimmer MedizinSysteme GmbH Junkersstraße 9 89231 Neu-Ulm, Germany Tel. +49 731. 9761-291 Fax +49 731.
- Page 44 The enPuls version 2.0 is developed according to the recognized standards of technology; the information on the intended use of the components is taken into account. The enPuls version 2.0 must not be operated near active HF surgery devices or magnetic resonance tomography that can cause high levels of electromagnetic interference.
- Page 45 Guidance and Manufacturing Declaration- Electromagnetic Emissions The device enPuls version 2.0 is intended for use in the electromagnetic environment specified below. The customer or user of the device enPuls version 2.0 should ensure that it is used in such environment. Emission Measurement...
- Page 46 Guidance and Manufacturing Declaration- Electromagnetic Immunity The device enPuls version 2.0 is intended for use in the electromagnetic environment specified below. The customer or user of the device enPuls version 2.0 should ensure that it is used in such environment.
- Page 47 Guidance and Manufacturing Declaration- Electromagnetic Immunity The device enPuls version 2.0 is intended for use in the electromagnetic environment specified below. The customer or user of the device enPuls version 2.0 should ensure that it is used in such environment. Immunity Test...
- Page 48 Manufacturer's EMC declaration Electromagnetic immunity to HF radio communication equipment Test Maximum Immunity Test Band Distance Frequency Service Modulation Energy Level (MHz) (MHz) (V/m) 380-390 TETRA 400 Pulse Modulation 18 Hz 430-470 GMRS 460, FRS 460 ± 5kHz Derivation 1kHz Sine 704-787 LTE Band 13, 17 Pulse Modulation...
- Page 52 Version 2.0 Instructions for Use Zimmer MedizinSysteme GmbH Junkersstraße 9 89231 Neu-Ulm, Germany Tel. +49 7 31. 97 61-291 Fax +49 7 31. 97 61-299 export@zimmer.de www.zimmer.de...













Need help?
Do you have a question about the enPuls and is the answer not in the manual?
Questions and answers