Zimmer enPuls Instructions For Use Manual
Hide thumbs
Also See for enPuls:
- User manual (46 pages) ,
- Operating instructions manual (50 pages) ,
- Instructions for use manual (52 pages)
Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Zimmer enPuls
- Page 1 Instructions for Use enPuls...
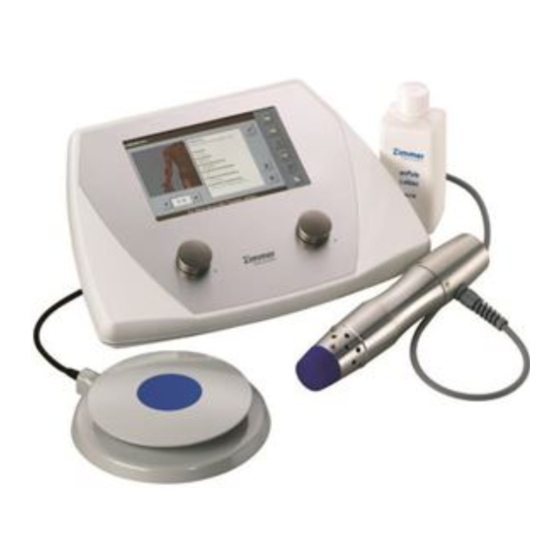
- Page 2 Diagrams Front View / Handpiece / Footswitch Front View Control unit Display Shockwave meter / counting direction Control for presetting number of shocks Control for resetting number of shocks Frequency display Control for adjusting frequency Energy level display Control for adjusting energy levels Handpiece Handpiece with 25 mm applicator head Air vents, front...
- Page 3 Illustrations Rear View of Device / Accessories Rear View Main switch Mains fuse Port for mains cable Port for footswitch Port for handpiece Standard rating plate Accessories Silicone cap standard 25 mm applicator head 15 mm applicator head 6 mm applicator head...
-
Page 4: Table Of Contents
These instructions contain general information on safety, operation, maintenance and care for owners and users of the enPuls shockwave therapy device. These instructions are valid at the date of printing. Requirements in terms of product and therapy technique are subject to constant change;... - Page 5 What does the The enPuls generates shockwaves by means of an ergonomic handpiece and enPuls do? delivers shockwaves via special applicators. It allows a maximum penetration depth of approximately 35 mm to be reached in human tissue.
-
Page 6: Explanation Of Symbols
Explanation of Symbols Danger / Warning In the User Manual, this symbol stands for Danger / Warning. Caution In the User Manual, this symbol stands for 'Caution' with regard to possible material damage. Handpiece port Footswitch port Follow instructions for use. Device type B: Protection level against electric shock Value of accessible fuses... -
Page 7: Quick Start Guide For The System
Positioning Position the handpiece on the selected treatment point/field. To avoid any handpiece/applicator friction on the skin, enPuls Lotion may first be applied to the treatment area if necessary. Note: When using enPuls Lotion, the applicator head must be covered with a silicone cap to protect it. -
Page 8: Start-Up Function Test
Start-up Function Test Start-up Note To start up the device, remove it from its transport case and place it on a suitable stable surface. This device is not designed for use in the case. Make sure that the main switch on the device is set to '0'. Note Before connecting the mains cable, make sure there is a suitable power outlet according to regulations, with the correct mains voltage. - Page 9 Switching on the Switch on enPuls using the rocker switch (I). device System test After power-up, enPuls runs a system test and if this is completed successfully it is then ready for use. Function test Performance Depress the footswitch briefly – the fan and shockwave generator will start immediately, whereby the shockwave generator has to operate at the frequency indicated on the display (5 Hz as default value).
-
Page 10: Description Of Functions
Description of Functions Shockwave meter In the basic setting and after a reset (5), the central meter (3) on the display counts the shocks delivered up to a maximum of 9999 and then starts again from zero. Counting is summative, that is, the meter counts upwards as long as the shockwave is activated by the footswitch (18) and stops if you remove your foot from the switch. - Page 11 Description of Functions Energy level Button (9) allows the energy level (that is, the impact hardness of the shockwave generator in the handpiece) to be set at 4 fixed levels. The function is only available when the shockwave is not activated. The amount of energy which is delivered and transferred to the applicator head at every impact is as follows: Level 1:...
-
Page 12: General Operating Instructions
When the shockwave is activated it is possible to work both stationarily on a single site or dynamically over an area. It is advisable to use enPuls Lotion (included in the accessories) in order to reduce friction on the skin. - Page 13 Note be replaced after a specific period of use as its functionality progressively decreases. Zimmer MedizinSysteme GmbH guarantees unrestricted use of at least 2 million shocks per shockwave generator. Wear on the shockwave generator varies. Depending on performance and frequency, sometimes far more than 2 million shocks can be delivered.
- Page 14 General Operating Instructions Applicator heads There are 3 different applicator heads available for treatment. Changing the To change the various applicator heads, hold the handpiece in applicator heads one hand and unscrew the applicator head from the handpiece with the other hand (anticlockwise). Screw the required head tightly into the handpiece (clockwise), until the black outside ring of the applicator head rests on the handpiece (there should no longer be any thread visible).
- Page 15 General Operating Instructions Footswitch Place the footswitch so that it can be reached easily during treatment. The footswitch control unit is non-directional so it is not necessary to align the footswitch exactly. To avoid damage please note that only slight pressure is needed or should/must be exerted on the switch using the front part of your foot, not the heel.
-
Page 16: Errors Troubleshooting
Errors Troubleshooting Failure or Check to ensure that the handpiece plug is properly connected to the device. malfunction of the It must be fully engaged. handpiece Check the helix cable of the handpiece for any mechanical damage. Irregular delivery of Possible cause 1: Wear of shockwave generator shockwaves / gebraucht... - Page 17 Errors Troubleshooting No response at Make sure that the mains plug is properly inserted in the power outlet and the main switch device connector is firmly plugged into the device port. Display remains dark Check mains cable for any damage. Check the power supply system and the power outlet.
-
Page 18: Maintenance Cleaning / Disinfection Disposal
You should also check the applicator domes for any wear, as described in Section 6. Note When using enPuls Lotion, it is essential to pull a silicone cap over the applicator head. If you do not use any protective cap, lotion can get inside the applicator head and handpiece which can lead to permanent soiling and malfunctioning. -
Page 19: Warnings
Warnings Users of the enPuls shockwave therapy device must be trained in how to use the system properly and have the appropriate skills. Any treatment instructions regarding treatment location, duration and strength require medical knowledge and should only be given by authorized physicians, therapists and health paraprofessionals. -
Page 20: Indications Contraindications
Indications Contraindications Indications enPuls is designed to treat superficial orthopedic and neurological problems such as the following: Tendinitis Bursitis Insertion tendopathy Calcaneal spurs Myofascial trigger points, etc. Contraindications vascular diseases present in or near the treatment area local infections in the treatment area... -
Page 21: Technical Data
Technical Data enPuls: Treatment system for electromagnetic generation/application of radial shockwaves in orthopedics/physiotherapy Length 330 mm Width 250 mm Height 185 mm Weight 5.4 kg Power supply 230 VAC / 50/60 Hz, 2.5 A Fuse 2.5 AT Conformity Protection class I / application class B... -
Page 22: Contents On Delivery / Accessories
Contents on delivery / Accessories Contents on delivery Control unit for enPuls Handpiece, complete 6 mm applicator head 15 mm applicator head 25 mm applicator head Silicone cap, standard enPuls Lotion Footswitch Mains cable Instructions for use Template Transport case... -
Page 23: Manufacturer's Declaration Of Electromagnetic Compatibility
Portable and mobile RF communication systems (e.g. mobile phones) may interfere with medical electrical equipment. enPuls should only be operated with the original mains cable specified in the list of contents delivered. Operating the device with any other mains cable can lead to increased emissions or reduced interference immunity of the device. - Page 24 Electromagnetic Compatibility Guidance and manufacturer’s declaration – Electromagnetic immunity The enPuls device is intended for use in the electromagnetic environment specified below. The customer or the user of the enPuls device should assure that it is used in such an environment.
- Page 25 Uninterrupted operation is not required with the use intended. Guidelines and manufacturer's declaration – electromagnetic interference immunity The device enPuls is intended for operation in the electromagnetic environment specified below. The customer or user of the enPuls should ensure that it is used in such an environment.
- Page 26 If the measured field strength in the location where the enPuls device is to be used exceeds the above compliance levels, the enPuls device should be monitored in order to ensure that it is functioning as intended. If unusual features are noticed, additional measures may be necessary such as re-orienting or relocating the enPuls device.
-
Page 27: Declaration Of Conformity Legal Information
Declaration of Conformity Legal Information Declaration of This product bears the EC marking 0123 in accordance Conformity with EU Medical Products Directive 93/42/EEC and meets the essential requirements of Annex I to this Directive. The product was developed, manufactured and tested using the quality management system according to DIN EN ISO 13485. - Page 30 Instructions for Use Zimmer MedizinSysteme GmbH Junkersstrasse 9 D-89231 Neu-Ulm Tel. +49 731. 97 61-291 Fax +49 731. 97 61-299 export@zimmer.de www.zimmer.de...












Need help?
Do you have a question about the enPuls and is the answer not in the manual?
Questions and answers