Zimmer enPuls Operating Instructions Manual
Hide thumbs
Also See for enPuls:
- Instructions for use manual (30 pages) ,
- User manual (46 pages) ,
- Instructions for use manual (52 pages)
Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Zimmer enPuls
- Page 1 Operating Instructions enPuls Version 2.0...
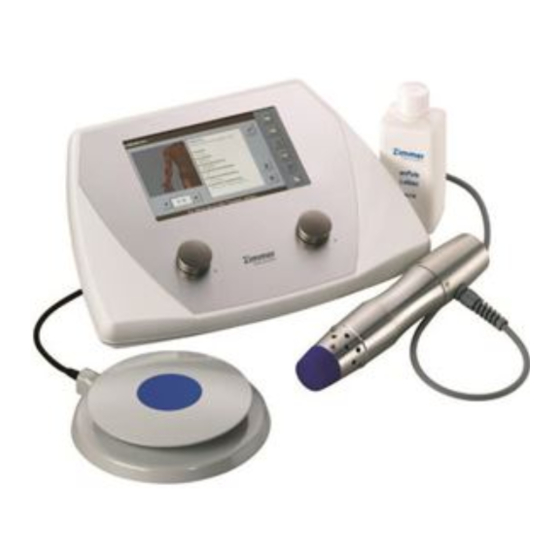
- Page 3 Figures Front view of device Control unit / Handpiece / Footswitch Control unit Selection and control Shock energy controller elements Touch pen in holder Display Frequency controller SD card slot Handpiece with applicator head 25 mm Handpiece Air vents, front Air vents with fan, rear Footswitch Footswitch...
- Page 4 Figures Front view of device Display Status bar Screen readouts Buttons on the screen Title bar Navigation bar...
- Page 5 Figures Rear view of device Switch and connector sockets Main switch Switch / Mains fuse Connector sockets Port for mains cable Port for footswitch Port for handpiece Serial number / manufacturer's plate...
- Page 6 Figures Accessories Silicone cap 25 mm applicator head 15 mm applicator head 6 mm applicator head...
-
Page 7: Table Of Contents
Rear view of device Figures – Switch and connector sockets Accessories Figures enPuls briefly 1.1. Summary 1.2. Quick operation instruction 1.3. How to use enPuls 1.4. Handpiece 1.5. Applicator heads 1.6. Footswitch Installation 2.1. Fitting the cables, starting the system 2.2. - Page 8 Scope of delivery - Accessories Manufacturer’s declaration of Electromagnetic Compatibility Valid for the enPuls V.2.0 devices These operating instructions are an integral part of the device. They must be stored with the device and kept accessible at all times for anyone authorised to operate this device.
- Page 9 Creation of shockwaves using an ergonomic handpiece and the What does transmittal of the shockwaves via special applicators. enPuls do? enPuls has a maximum penetration depth of about 35 mm in human tissue. An electromagnetic field is generated via a coil in the back of the How are handpiece.
-
Page 10: Enpuls Briefly
Applicator out and screw this correctly onto the handpiece. Position the handpiece on the selected treatment point / field. To avoid Positioning handpiece / any friction on the skin, enPuls lotion may first be applied onto the applicator treatment area. - Page 11 1.2. Quick operation instruction When using lubricants, the applicator head must be covered with a Caution silicone cap to protect it. Adjust the shock energy using the left controller. Setting the shock energy Depress the footswitch to start the treatment.
-
Page 12: How To Use Enpuls
When the shockwave is activated, it is possible to work either steadily on a single site or dynamically over an area. It is advisable to use enPuls lotion (included in the accessories) in order to reduce friction on the skin. -
Page 13: Handpiece
Zimmer MedizinSysteme GmbH guarantees unrestricted use of at least 2 million shocks per shockwave generator. Wear on the shockwave generator varies. Depending on performance and frequency, sometimes far more than 2 million shocks can be delivered. -
Page 14: Applicator Heads
1.5. Applicator heads There are 3 different applicator heads available for treatment. To change the various applicator heads, hold the handpiece in one hand Changing and unscrew the applicator head from the handpiece with the other hand applicator heads (anticlockwise). -
Page 15: Footswitch
1.6. Footswitch Place the footswitch so that it can be reached easily during treatment. The footswitch control unit is multi-directional so it is not necessary to align the footswitch exactly. To avoid damage, please note that only slight pressure needs to be exerted on the switch. -
Page 16: Installation
Fitting the cables, starting the system Note: Before starting up the system, remove enPuls from its transport case. Do not operate the device while it is in the case. Ensure that enPuls is placed on a stable surface. Make sure that the main switch on the device is set to '0'. -
Page 17: Settings
Installation 2.2. Settings Note: Changes to the default settings can only be made from the start screen. Press button “Settings” to open the Settings screen Figure 1 Press this button to open the menu to select the language. The language Language is selected by pressing on the appropriate row in the pull down menu. - Page 18 Installation 2.2. Settings Audio / graphic settings Option to adjust the brightness of the screen lighting. Brightness Option to adjust the volume of the signals when activating the control Volume fields. Adjust the volume using the two arrow keys. The counter status for the handpiece that is currently connected, is Handpiece shown in this display field.
-
Page 19: Sd-Card
SD-Card User-defined settings and the treatment recommendation list are saved on the SD card. Note: If the SD card is not inserted, the message ‘SD card not found’ appears when the 'Favourites' and 'Memory' buttons are pressed. The ‘Therapy‘ button is not shown. Deactivate the message by pressing the button “OK”... -
Page 20: Treatment Screen
Treatment screen The title bar shows the name of the current programme. Title bar The status bar shows the information about the current status of the Status bar treatment. If the treatment is not active, it shows the word ‘Ready' and if treatment is running the text 'Active' appears. - Page 21 Also the count direction (increasing in this case) is shown. Pressing the “Number of shocks” field opens the Input window, defining pre-selection. Note: enPuls offers two options for shock delivery: Shock delivery without For shock delivery with no pre-selected number of shocks, the device pre-selecting the shock does not end the treatment.
- Page 22 Treatment screen Shock delivery with For shock delivery with a pre-selected shock number, the device ends pre-selection of the the treatment once the pre-selected number of shocks has been number of shocks reached. The footswitch is deactivated and shock delivery is no longer possible. The treatment can be continued by resetting the current number of shocks or by adjusting the pre-selection.
-
Page 23: Favourites And Memory List
Favourites and Memory list 5.1. Saving modified programmes Programmes can be stored either in the Favourite list or the Memory list. For saving the programme enter the programme name using the Programme name keyboard Press the “Favourites” button to open the Favourites list and Favourites automatically save the programme. -
Page 24: Retrieving And Editing Favourites List And The
Favourites and Memory list 5.2. Retrieving and editing Favourites list and the Memory list Note The following steps to edit the Favourites list correspond exactly to those used to edit the Memory list. Individual saved programmes are listed in Favourites or Memory list. From here they can be: 1. - Page 25 Favourites and Memory list 5.2. Retrieving and editing Favourites list and the Memory list Press the ‘Edit’ button to open the 'Edit Favourites' screen Editing Activate the desired programme by pressing the appropriate row. You are now able to Move or •...
-
Page 26: Description Of The Selection Keys
Description of the selection keys Press the button to open the screen to save a programme The “Save” button can only be pressed from the treatment screen. Can be used to reverse the counting direction. Pressing the key reset the current number of shocks •... - Page 27 Description of the selection keys Press the button to reject the changes made. Activation of the „+“ button increase the pulse rate in 1000 increments, activation of the „-“ button reduces the number of the pulses in 1000 steps. Activation of the „+“ button increase the pulse rate in 100 increments, activation of the „-“...
-
Page 28: Treatment Recommendation
Treatment recommendation The Treatment recommendation menu helps you to select the treatment. The treatment can be selected using the body region menu or the treatment recommendation list. Press the “Therapy” button to open the “Selecting by body region” Therapy window. Select a body region by touching a circle. - Page 29 Treatment recommendation Press the “List” button to open the list. Selecting by treatment recommendation list Further steps will only be described once, as the selection though Body Note: Parts or List opens a similar list. The selection of a treatment recommendation opens another window Selecting detailed showing the detailed symptoms.
- Page 30 Treatment recommendation After selecting the detailed symptoms, another window opens showing Treatment information detailed treatment and treatment information. Press the “Therapy” button to open the treatment screen with the Selecting the programme. treatment programme...
- Page 31 Treatment recommendation Press the “Info” button to open the window with the treatment Retrieving treatment information. information...
-
Page 32: Medical Information
Medical information 8.1. Indications • Radial and ulnar epicondylitis • Calcific tendonitis of the shoulder / shoulder problems • Status post muscular injuries • Chronic patellar tendonitis • Jumper's knee • Achillodynia • Plantar fasciitis • Heel spurs • Myofascial trigger point treatment e.g. neck •... - Page 33 Medical information 8.2. Contraindications • vascular diseases present in or near the treatment area • local infections in the treatment area • around malignant or benign tumours • directly on cartilage surfaces or near the small facet joints of the spinal column •...
-
Page 34: General Information
General information 9.1. Explanation of symbols In the Operating Instructions, this symbol stands for Danger / Warning. Danger / Warning In the Operating Instructions this symbol stands for 'Caution' with regard Caution to possible damage to property. Port for handpiece Port for footswitch Follow Operating Instructions Instrument type BF (according EN60601-1):... -
Page 35: Warnings
General information 9.2. Warnings Users of the enPuls shockwave treatment device must be trained in how to use the system properly and have the appropriate skills. Any treatment instructions regarding treatment location, duration and strength require medical knowledge and should only be given by authorised doctors, therapists and health paraprofessionals. -
Page 36: Technical Data
General information 9.3. Technical data Treatment system for electromagnetic generation / application of radial enPuls Version 2.0: shockwaves in orthopaedics and physiotherapy. L 322 mm / W 235 mm / H 130 mm Dimensions 2.7 kg Weight 100–240 VAC / 50/60 Hz, 220 VAC / 60 Hz... -
Page 37: Technical Information
General information 9.4. Technical information As the manufacturer Zimmer MedizinSysteme can only be responsible for the safety and reliability under the following circumstances: • if the device is operated from an approved, grounded wall socket and the electrical installation conforms to DIN VDE 0100 Part 710 •... -
Page 38: Ce Marking / Legal Information
General information 9.5. CE Marking / Legal information CE Marking This product bears the marking 0123 in accordance with EU Medical Products Directive 93/42/EEC and meets the essential requirements of Annex I to this Directive. The product was developed, manufactured and tested using the quality management system according to DIN EN ISO 13485. -
Page 39: Maintenance
Maintenance Separate servicing is not required for this product. Before starting any maintenance or cleaning, the device must always be switched off at the main switch and the plug pulled out. You should also check the applicators domes for any wear, as described in chapter 1.5. - Page 40 Maintenance Generating mechanical shockwave energy causes a considerable build Monitoring the up of heat in the handpiece. To avoid shortening the lifetime of the handpiece handpiece, a temperature switch has been integrated. This triggers an temperature internal switch-off, if the temperature becomes too high, forcing the handpiece to cool.
-
Page 41: Troubleshooting
Troubleshooting Check to ensure that the handpiece plug is properly connected to the Failure or malfunction device. of the handpiece It must be fully engaged. Check the cable of the handpiece for any mechanical damage. Possible cause 1: Wear of applicator head Irregular delivery Difficult to move due to wear of shockwaves /... - Page 42 Troubleshooting Make sure that the mains plug is properly inserted in the power outlet No response at and the device connector is firmly plugged into the device port. main switch / display remains Inspect the mains cable for damage. dark Check the power supply and the power plug.
-
Page 43: Function Test
Function test enPuls runs a self-test that checks all internal components after it is switched on. An error message is shown in case of faults. In addition, a function test shall be made as follows. This test shall be made monthly or in case of doubt about the proper function of the device. -
Page 44: Error Messages
Error Messages Check that the handpiece is correctly connected In the status bar the message 'Handpiece not found' appears. Generating mechanical shockwave energy causes a considerable build Monitoring up of heat in the handpiece. To avoid shortening the lifetime of the the handpiece handpiece, a temperature switch has been integrated. -
Page 45: Scope Of Delivery, Accessories
Scope of delivery, Accessories Control unit enPuls Version 2.0 Scope of delivery Handpiece, complete with a 15 mm applicator head 6 mm applicator head 15 mm applicator head 25 mm applicator head Silicone caps enPuls lotion Footswitch Mains cable Operating instructions... -
Page 46: Manufacturer's Declaration Of Electromagnetic Compatibility
Portable and mobile RF communication systems (e.g. mobile phones) may interfere with medical electrical equipment. enPuls Version 2.0 should only be operated with the original mains cable specified in the list of contents delivered. Operating the device with any other mains cable can lead to increased emissions or reduced interference immunity of the device. - Page 47 Guidance and manufacturer’s declaration – Electromagnetic immunity The enPuls Version 2.0 device is intended for use in the electromagnetic environment specified below. The customer or the user of the enPuls Version 2.0 device should assure that it is used in such an environment.
- Page 48 Guidelines and manufacturer's declaration – electromagnetic interference immunity The device enPuls Version 2.0 is intended for operation in the electromagnetic environment specified below. The customer or user of the enPuls Version 2.0 should ensure that it is used in such an environment.
- Page 49 Version 2.0 device is to be used exceeds the above compliance levels, the enPuls Version 2.0 device should be monitored in order to ensure that it is functioning as intended. If unusual features are noticed, additional measures may be necessary such as re-orienting or relocating the enPuls Version 2.0...
- Page 50 Version 2.0 Operating Instructions Zimmer MedizinSysteme GmbH Junkersstraße 9 D-89231 Neu-Ulm Tel. +49 731. 97 61-291 Fax +49 731. 97 61-299 export@zimmer.de www.zimmer.de...












Need help?
Do you have a question about the enPuls and is the answer not in the manual?
Questions and answers