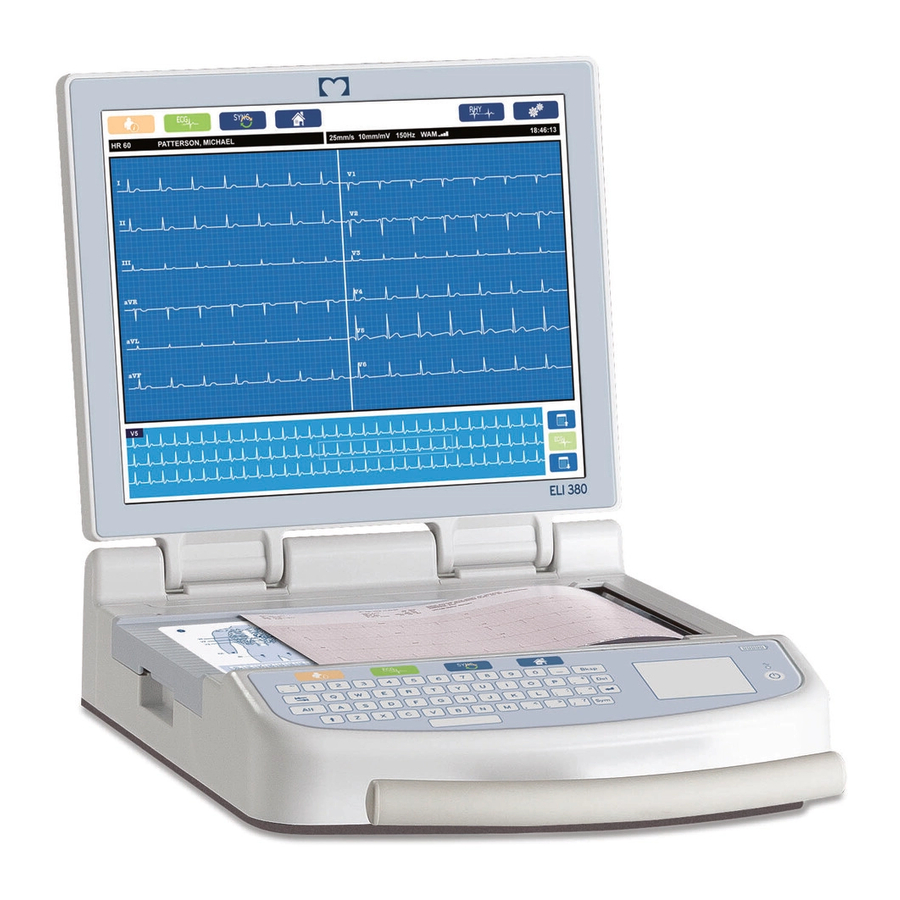
Mortara ELI 380 User Manual
Late potential
Hide thumbs
Also See for ELI 380:
- Service manual (108 pages) ,
- User manual (93 pages) ,
- Quick reference manual (2 pages)
Table of Contents

Summary of Contents for Mortara ELI 380
-
Page 1: User Manual
REF 9515-189-51-ENG Rev A1 ELI 380 Late Potential ADDENDUM TO ELI 380 USER MANUAL Manufactured by Mortara Instrument, Inc., Milwaukee, Wisconsin U.S.A. CAUTION: Federal law restricts this device to sale by or on the order of a physician. - Page 2 This document contains confidential information that belongs to Mortara Instrument, Inc. No part of this document may be transmitted, reproduced, used, or disclosed outside of the receiving organization without the express written consent of Mortara Instrument, Inc. Mortara is a registered trademark of Mortara Instrument, Inc. ELI is a trademark of Mortara Instrument, Inc. v2.2.0...
- Page 3 Physician Practice: orderspc.us@mortara.com U.S. Distribution: orderspc.us@mortara.com European Union Representative Mortara Instrument Germany Bonifaciusring 15 45309 Essen Mortara Instrument Europe, s.r.l. Germany (European Headquarters) Tel: +49.201.18 55 69 70 Via Cimarosa 103/105 Fax: +49.201.18 55 69 77 40033 Casalecchio di Reno (BO)
- Page 4 Failure to do so may cause undue failure and possible health hazards. Equipment Identification Mortara Instrument, Inc. equipment is identified by a serial and reference number on the back of the device. Care should be taken so that these numbers are not defaced.
-
Page 5: Warranty Information
Product/s. If Mortara should be found liable to anyone under any theory (except the expressed warranty set forth herein) for loss, harm, or damage, the liability of Mortara shall be limited to the lesser of the actual loss, harm, or damage, or the original purchase price of the Product/s when sold. - Page 6 WARRANTY INFORMATION...
- Page 7 Reference the ELI™ 380 User Manual for all warnings. Before attempting to use the device, the user must read and understand the contents of the user manual and any accompanying documents. Caution(s) Reference the ELI 380 User Manual for all cautions.
- Page 8 EQUIPMENT SYMBOLS AND MARKINGS Symbol Delineation Attention, consult accompanying documents Do not dispose as unsorted municipal waste. Per European Union Directive 2002/96, requires separate handling for waste disposal according to national requirements Reference the ELI 380 user manual for further symbols...
-
Page 9: Table Of Contents
TABLE OF CONTENTS INTRODUCTION AND OPERATION SECTION 1 Manual Purpose ................................1 Late Potential (SAECG) ..............................1 Signal Averaging ................................2 Patient Prep ..................................3 Format ....................................4 Relearn ..................................... 4 End Test ................................... 4 Starting a New Test on the Same Patient ......................... 4 Exiting the Signal Averaging Application ........................ - Page 10 TABLE OF CONTENTS viii...
-
Page 11: Introduction And Operation Section
Late Potential (SAECG) The ELI 380 Late Potential (SAECG) option allows for patient ID entry and the acquisition, analysis, and printout of signal-averaged ECGs in order to detect ventricular late potentials. When acquiring late potentials, orthogonal bipolar leads XYZ are most commonly used. -
Page 12: Signal Averaging
The noise level can be lower than 1 μV after averaging 100 to 500 cycles. (1 μV = 1/100 of a millimeter on regular ECG paper.) The ELI 30 incorporates a state-of-the-art Mortara amplifier which guarantees low noise and high quality signal acquisition. -
Page 13: Patient Prep
Careful skin preparation, a relaxed patient, and the use of good quality electrodes are essential to a good quality signal. Follow the skin preparation procedure explained in the ELI 380 User Manual. Prepare the electrodes and place them in the following positions: ... -
Page 14: Format
Start. The associated demographic information. After the first test has been completed, select ELI 380 resets the QRS or acquired beat numbers and starts a new test. Follow the steps explained in Acquiring Late Potentials. Exiting the Late Potentials Application... -
Page 15: Acquiring Late Potentials
to go back to the real-time display, and select Start to begin another test. Select The ELI 380 will automatically end the test when the user-defined criteria are met: Total number of beats. Target noise level. - Page 16 SECTION 1 Following Acquisition, the clinician or technician user can choose to do one of the following: Select in the upper left corner to modify patient demographic information Select Erase to erase the test, which will then bring the user back to the home screen ...
- Page 17 SECTION 1 Upon selection of a Late Potential test from the Directory, the following may be completed using the listed steps: Edit patient demographic information: Select the button When prompted, “Edit current late potentials demographics?” select Yes Modify demographics and select OK to save or Cancel to return to the report preview ...
-
Page 18: Specifications
SECTION 1 Specifications Feature Specification 0.05 – 300 Hz Frequency Response Analysis sampling frequency 1000 s/s Input signal resolution 0.9375 µV LSB Averaged signal resolution 5 nV LSB Vector Magnitude signal resolution 5 nV LSB 20 – 300 bpm Heart rate range 0.12 –...














Need help?
Do you have a question about the ELI 380 and is the answer not in the manual?
Questions and answers