Table of Contents

Summary of Contents for Mortara ELI 230
-
Page 1: User Manual
REF 9515-175-50-ENG Rev G1 ELI 230 12-LEAD RESTING ELECTROCARDIOGRAPH USER MANUAL Manufactured by Mortara Instrument, Inc., Milwaukee, Wisconsin U.S.A. CAUTION: Federal law restricts this device to sale by or on the order of a physician. - Page 2 Failure to do so may cause undue failure and possible health hazards. Equipment Identification Mortara Instrument, Inc. equipment is identified by a serial and reference number on the back of the device. Care should be taken so that these numbers are not defaced.
-
Page 3: Warranty Information
Product/s. If Mortara should be found liable to anyone under any theory (except the expressed warranty set forth herein) for loss, harm, or damage, the liability of Mortara shall be limited to the lesser of the actual loss, harm, or... - Page 4 WARRANTY INFORMATION EXCEPT AS SET FORTH HEREIN WITH RESPECT TO REIMBURSEMENT OF LABOR CHARGES, A PURCHASER’S SOLE EXCLUSIVE REMEDY AGAINST MORTARA FOR CLAIMS RELATING TO THE PRODUCT/S FOR ANY AND ALL LOSSES AND DAMAGES RESULTING FROM ANY CAUSE SHALL BE THE REPAIR OR REPLACEMENT OF DEFECTIVE PRODUCT/S TO THE EXTENT THAT THE DEFECT IS NOTICED AND MORTARA IS NOTIFIED WITHIN THE WARRANTY PERIOD.
- Page 5 • Only use parts and accessories supplied with the device and/or are available through Mortara Instrument, Inc. • Patient cables intended for use with the device include series resistance (9 Kohm minimum) in each lead for defibrillation protection.
- Page 6 The ELI 230 is a medical device that has been designed to be connected to other devices for the purpose of receiving and transmitting data. Certain measures must be taken to prevent the risk of excessive electric current flow through the operator or patient when connected: •...
- Page 7 • The rechargeable internal battery is a sealed lead-acid type and it is totally maintenance free. If the battery appears to become defective, refer to Mortara Instrument Service Department. • Do not pull or stretch patient cables as this could result in mechanical and/or electrical failures. Patient cables should be stored after forming them into a loose loop.
- Page 8 USER SAFETY INFORMATION FCC Compliance Statement for the WAM In the United States use of this device is regulated by the Federal Communications Commission (FCC). The WAM with its antenna complies with FCC’s RF exposure limits for general population/uncontrolled exposure. FCC Warning (Part 15.21): Changes or modifications not expressly approved by the party responsible for compliance could void the user’s authority to operate the device.
- Page 9 • The WAM will automatically turn off after being disconnected from the patient. This will happen regardless of ELI 230 battery/AC power state. • The display of absent waveform display while using the AM12 acquisition module could be due to an improper auto-calibration.
- Page 10 USER SAFETY INFORMATION • After operating the device using battery power, always reconnect the power cord. This ensures that the batteries will be automatically recharged for the next time you use the device. A light next to the on/off switch will illuminate indicating that the device is charging.
- Page 11 EQUIPMENT SYMBOLS AND MARKINGS Device Symbol Delineation Attention, consult accompanying documents Alternating current Battery Charging Indicator ECG Patient Cable Input Protective earth Defibrillator-proof type CF applied part ON/OFF (power) Continuous Rhythm 12-Lead ECG Do not dispose as unsorted municipal waste. Per European Union Directive 2002/96, requires separate handling for waste disposal according to national requirements Antenna...
- Page 12 EQUIPMENT SYMBOLS AND MARKINGS Package Symbol Delineation This side up Fragile Keep Dry Keep Away from Heat Acceptable Temperature Range Contains Non-spillable Battery...
-
Page 13: Cleaning The Device
GENERAL CARE Precautions • Turn off the device before inspecting or cleaning. • Do not immerse the device in water. • Do not use organic solvents, ammonia-based solutions, or abrasive cleaning agents which may damage equipment surfaces. Inspection Inspect your equipment daily prior to operation. If you notice anything that requires repair, contact an authorized service person to make the repairs. - Page 14 GENERAL CARE...
- Page 15 See appropriate EMC table for recommended separation distances between the radio equipment and the device. The use of accessories, transducers, and cables other than those specified by Mortara Instrument may result in increased emissions or decreased immunity of the equipment.
- Page 16 ELECTROMAGNETIC COMPATIBILITY (EMC) Guidance and Manufacturer’s Declaration: Electromagnetic Emissions The equipment is intended for use in the electromagnetic environment specified in the table below. The customer or the user of the equipment should ensure that it is used in such an environment. Emissions Test Compliance Electromagnetic Environment: Guidance...
- Page 17 ELECTROMAGNETIC COMPATIBILITY (EMC) Guidance and Manufacturer’s Declaration: Electromagnetic Immunity The equipment is intended for use in the electromagnetic environment specified in the table below. The customer or the user of the equipment should ensure that it is used in such an environment. IEC 60601 Test Compliance Emissions Test...
- Page 18 ELECTROMAGNETIC COMPATIBILITY (EMC) Recommended Separation Distances Between Portable and Mobile RF Communications Equipment and the Equipment The equipment is intended for use in the electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the equipment can help to prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the equipment as recommended in the table below, according to the maximum output power of the communications equipment.
-
Page 19: Table Of Contents
Audience ..................................1 Indications for Use ................................ 1 System Description ................................ 2 Figure 1-1, ELI 230 System Illustration ........................3 Figure 1-2, ELI 230 Left Side ............................4 Figure 1-3, ELI 230 Rear............................... 4 Figure 1-4, ELI 230 Base .............................. 5 Figure 1-5, ELI 230 Display Overview ......................... - Page 20 TABLE OF CONTENTS RECORD AN ECG SECTION 3 Patient Preparation ............................... 21 Patient Hookup ................................21 Patient Demographic Entry ............................23 ECG Acquisition, Printing, Storage ..........................23 Acquisition ..............................23 Printing ................................. 25 Storage ................................25 Acquiring an ECG using the WAM ..........................26 Acquiring Rhythm Strips .............................
-
Page 21: Introduction Section
This manual is intended to provide the user with information about: • Using and understanding the ELI™ 230 electrocardiograph, the function keys, and the display screen. • Preparing the ELI 230 for use. (Section 2) • Acquiring, printing, and storing an ECG. (Section 3) •... -
Page 22: System Description
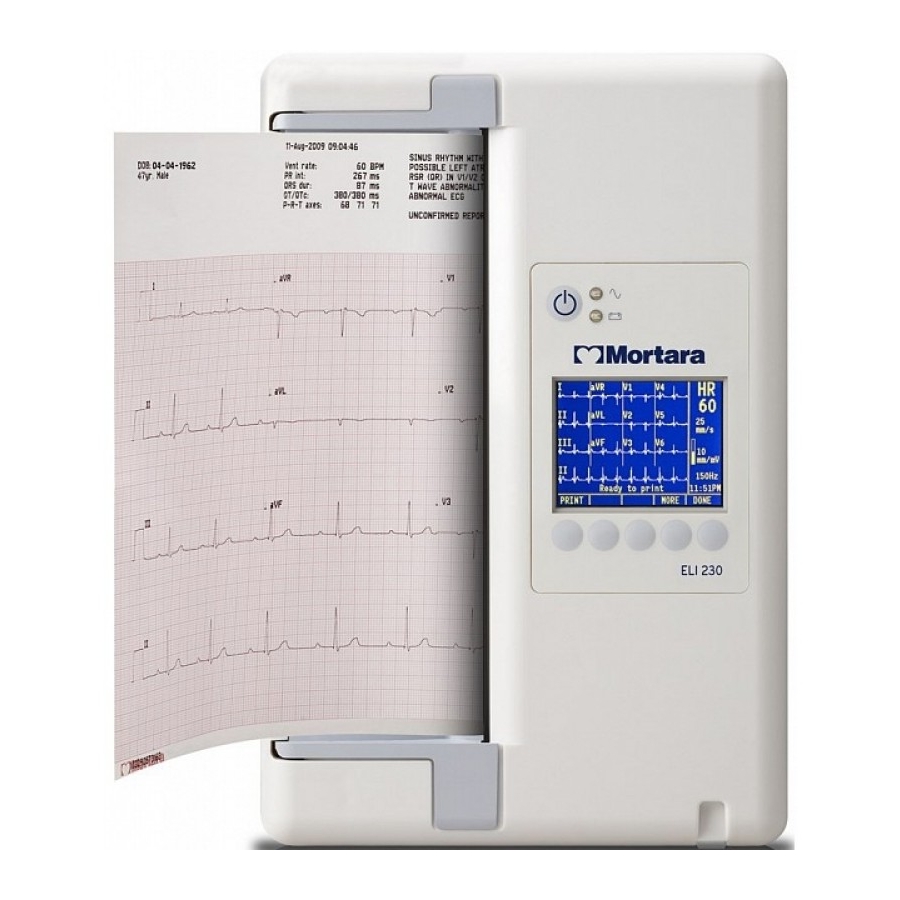
SECTION 1 System Description The ELI 230 is a 12-lead diagnostic electrocardiograph used for acquiring, viewing, and printing of adult and pediatric 12-lead ECG data. The device is optionally equipped with Mortara Instrument’s VERITAS™ resting ECG interpretation algorithm with age and gender specific criteria. If this option is enabled (see Section 4) the VERITAS algorithm can provide an over-reading physician with a silent second opinion through diagnostic statements output on the ECG report. - Page 23 SECTION 1 ELI 230, System Illustration Figure 1-1...
- Page 24 SECTION 1 ELI 230, Left Side Figure 1-2 ELI 230, Rear Figure 1-3...
- Page 25 SECTION 1 ELI 230, Base Figure 1-4...
-
Page 26: Function Keys
SECTION 1 ELI 230, Display Overview Figure 1-5 Heart Rate Speed Best 10 Gain Filter Clock Function Key Labels Function Keys Function Keys Function keys activate the liquid crystal display (LCD) label above each function key. LCD labels/functions change depending upon the screen displayed. If the label is blank, the function key is not active. - Page 27 SECTION 1 The ELI 230 features a ¼ VGA 320 x 240 pixel LCD color display for valuable preview of ECG waveform, function key labels, and other parameters as explained below: Heart Rate (HR): When a patient is connected to the electrocardiograph, his/her HR is displayed in real time. The HR is the average ventricular rate measured over an average of the patient’s last five beats.
-
Page 28: Specifications
High-performance baseline filter; AC interference filter 50/60 Hz; low- pass filters 40 Hz, 150 Hz, or 300 Hz Optional Functions Optional Mortara VERITAS resting ECG interpretation with age and gender specific algorithm Paper Thermal roll paper; 210 mm (8.25”) wide Thermal Printer Computer-controlled dot array;... -
Page 29: Accessories
SECTION 1 Accessories Part Numbers Description 9100-029-50 PAPER CASE ELI 230 ROLL W/ HEADER 210mm 9293-048-54 AM12 PATIENT CABLE ASSEMBLY 30012-019-54 WIRELESS ACQUISITION MODULE (WAM) 9300-036 ELECTRODES RESTING 24mm SUCTION PK/6 9300-037 ELECTRODE RESTING CLAMP IEC PK/4 IEC 9325-001-50 ELECTRODE CLIP 4mm SET OF 10... -
Page 30: Electrodes
Part Number Description 9300-032-50 ECG MONITORING ELECTRODES CASE 300 9300-033-51 ELECTRODE RESTING TAB BOX/500 9300-033-52 ELECTRODE RESTING TAB CASE/5000 047029 ULTRA II RESTING TAB ELECTRODES 015-0630-00 BLUE MAX RESTING TAB ELECTRODES Contact your dealer or go to www.mortara.com for more information. -
Page 31: Equipment Preparation Section
SECTION 2 Connecting the Acquisition Module Connect the AM12™ to the USB port on the front end of the device. The ELI 230 will automatically convert to the AM12 acquisition module. When using the WAM™ (wireless acquisition module) for ECG acquisition, the connector is not required. Refer to Using the WAM (Wireless Acquisition Module) in this section. -
Page 32: Loading Paper
WARNING: Risk of injury to fingers in paper tray door or platen drive mechanisms. NOTE: For proper performance of thermal writer, be certain to use Mortara recommended thermal paper. -
Page 33: Applying Power
Applying Power 1. Plug the power cord into an AC wall outlet and into the back of the ELI 230. (Reference Figure 1-3.) Device powers on automatically and cannot be turned off when AC is connected (device can be put into standby mode). -
Page 34: Setting Date And Time
Using the WAM (Wireless Acquisition Module) NOTE: The ELI 230 must be configured at the factory for use with the WAM. Select MORE, followed by CONFIG to determine the device’s setting. “Wireless Option Available” will display if the ELI 230 is configured to work with the WAM. -
Page 35: Wam With Lead Wires
Acquiring a rhythm strip Approved Battery Models Description Manufacturer Part Numbers Alkaline, AA-type, 1.5V Various Various WARNING: Use of other cells may present a risk of fire or explosion. To order additional supplies, contact a Mortara Instrument customer service representative. -
Page 36: Wam Specifications
SECTION 2 WAM Specifications Feature Specification* Instrument Type 12-lead wireless acquisition module for resting ECG Input Channels 12-lead signal acquisition and transmission ECG Leads Transmitted I, II, III, aVR, aVL, aVF, V1, V2, V3, V4, V5, and V6 WAM Transmission Protocol Bidirectional and frequency hopping;... -
Page 37: Battery Installation
SECTION 2 Battery Installation The WAM is powered with a single AA battery. When the battery contains sufficient voltage to operate and the patient is properly connected, an LED on the front of the WAM will appear solid green indicating proper pairing and communication with the electrocardiograph. -
Page 38: Pairing The Wam With Eli 230
Select 4 Service. • Select Yes. • Select 2 WAM Pairing. • Place the WAM (powered off) on top of the ELI 230. • Select START, then turn WAM on. • A successfully paired message will display. • Select DONE. -
Page 39: Cleaning The Wam And Ecg Lead Wires
SECTION 2 Cleaning the WAM and ECG Lead Wires 1. Remove all lead wires and the power source from the WAM before cleaning. 2. For general cleaning, use a soft, lint-free cloth lightly moistened with a mild soap and water solution. Wipe and air dry. - Page 40 SECTION 2...
-
Page 41: Record An Ecg Section
RECORD AN ECG SECTION 3 Patient Preparation Before attaching the electrodes, assure the patient fully understands the procedure and what to expect. • Privacy is very important in assuring the patient is relaxed. • Reassure the patient that the procedure is painless and that the electrodes on their skin are all that they will feel. - Page 42 SECTION 3 For accurate V-lead placement and monitoring, it is important to locate the 4 intercostal space. The 4 intercostal space is determined by first locating the 1 intercostal space. Because patients vary with respect to body shape, it is difficult to palpate the 1 intercostal space with accuracy.
-
Page 43: Patient Demographic Entry
ECG acquisition and rhythm strip printing can also be performed at the WAM (wireless acquisition module) or the AM12 acquisition module. To use either acquisition module with the ELI 230, refer to the WAM user manual or the AM12 short-form instruction card. - Page 44 SECTION 3 Examine the display for any of the following notification messages: • Leads Off –displays when patient is not connected. • Lead Fault –displays faulty lead(s). Re-prep and replace electrode(s) if necessary to obtain satisfactory waveform(s). (See Patient Preparation.) •...
-
Page 45: Printing
Storage The ELI 230 will automatically store up to 20 ECGs in its internal memory; however, the ECG records must be transferred via USB memory stick to a PC running ELI Link to be reviewed. Starting with 20 and counting down to 0, the device will display the number of remaining storage slots available just below the display sweep speed on the right side of the display in acquired ECG view. -
Page 46: Acquiring An Ecg Using The Wam
Begin routine rhythm strips by connecting the patient to the ELI 230 and entering the patient data. Once completed, select DONE to return to the real-time ECG view. Select RHY to begin rhythm printing. You can also acquire a rhythm printout by selecting RHY without entering the patient data. -
Page 47: Transfer To A Usb Memory Stick
Transfer to a USB Memory Stick The user can transfer all the ECG records from the ELI 230 to an external USB memory stick at any time. Using a PC, create a directory on the USB memory stick called “Records.” When done, plug the USB memory stick into the USB port on the ELI 230 (same port as used for the AM12 acquisition module). - Page 48 SECTION 3...
-
Page 49: Accessing Configuration Menus
SYSTEM SETTINGS SECTION 4 Accessing Configuration Menus The configuration pages define all operational conditions that do not change on a daily or patient-to-patient basis. Once you set these default conditions, you will rarely need to use the configuration screens again. To access the configuration menus: 1. - Page 50 SECTION 4 The following chart summarizes the configuration parameters and the available options for each field. Summary of Configuration Menus Configuration Parameter Definition Software Version Displays software version on printout and in configuration menu Language Software language availability Battery Timeout 10, 20, or 30 minutes Time Mode 12 hour or 24-hour clock...
-
Page 51: Configuration Settings
AC Filter The ELI 230 removes 60 Hz or 50 Hz interference. The setting you select depends on the line frequency in your country. Always use the 60 Hz setting in the U.S. If AC interference is present, check to see that the proper AC... - Page 52 Configure to 5 mm/s, 10 mm/s, 25 mm/s, or 50 mm/s. Interpretation Option The ELI 230 automatically analyzes ECGs and prints the optional interpretation on the ECG printout. This setting allows you to select or suppress the “interpretive” text on the ECG printout.
- Page 53 SECTION 4 Auto-Print ECG Defines whether or not the ELI 230 will automatically print the ECG after acquisition. If the selected configuration option is set to No, a manual printout is possible. Display Format Defines the default display format in either 4+4 or 6+6. Regardless of the display format selected, 10 seconds of 12 leads are always acquired.
- Page 54 SECTION 4 ECG Capture Up to one minute accumulated ECG data can be acquired internally for use with the Best 10 feature. The device automatically selects the best 10 seconds from within the one-minute buffer. Users can switch between BEST 10 or LAST 10 by selecting BEST10 or LAST10 from acquired ECG view. Storage Warning Determines whether a storage warning will display when the electrocardiograph is near the maximum storage level.
-
Page 55: Maintenance And Troubleshooting
Test Operation After cleaning and inspecting the ELI 230, proper operation of the unit may be confirmed by using an ECG simulator to acquire and print a standard 12-lead ECG of known amplitude. Printing should be dark and even across the page. -
Page 56: Recommendations To Biomedical Staff
For improved battery life, keep the electrocardiograph plugged in when not in use. The sealed lead-acid battery will provide optimum life when the unit is fully charged after each use. The ELI 230 will charge a depleted battery to 85% of its capacity in approximately 6 hours or less...













Need help?
Do you have a question about the ELI 230 and is the answer not in the manual?
Questions and answers
The AC light is on but the machine refuses to turn on