Summary of Contents for St. Jude Medical 3077
- Page 1 MODEL 3077 Single Chamber (SSI) External Pulse Generator User’s Manual 5I-17-024X-B-05 © Osypka Medical 2011...
-
Page 3: Table Of Contents
Possible Complications Precautionary Measures and Warnings Patient Safety Electromagnetic Compatibility Use and Application of MODEL 3077 Design Connecting Temporary Stimulation Leads 9.2.1 Lead Types ... - Page 4 MODEL 3077 – User’s Manual 4 / 32 5I-17-024X-B-05...
-
Page 5: Preface
Unpack the product and carefully check to see if any damage has occurred during shipment. Check to see if everything was delivered as listed on the shipping list. Please inform St. Jude Medical immediately if something is missing or damaged. Claims that are filed afterwards will not be considered. -
Page 6: Product Description
Refer to applicable sections of this manual for more detailed information on the use and maintenance of MODEL 3077. The technical data is summarized at the end of this manual. -
Page 7: Indication
3 Indication In conjunction with a stimulation lead system, MODEL 3077 can be used whenever temporary atrial or ventricular stimulation is indicated. MODEL 3077 can be employed for therapeutic as well as diagnostic purposes or be used prophylactically. Some specific indications for temporary stimulation are: ... -
Page 8: Contraindication
4 Contraindication There are no contraindications with regards to the use of MODEL 3077 for temporary cardiac stimulation for therapy and prevention of arrhythmia. The state of health of the patient, however, can restrict the choice of operational mode and stimulation parameters. -
Page 9: Possible Complications
MODEL 3077 – User’s Manual 5 Possible Complications When using an external pacemaker such as MODEL 3077, the following complications can arise and may lead to adverse events: Complication Result / Adverse Event Infection Sepsis Thrombosis and pulmonary Death embolism... -
Page 10: Precautionary Measures And Warnings
13. If MODEL 3077 is to be used for a long period of time on a patient, then the stimulation threshold should be checked from time to time. Initially, it should be checked after a few hours, then daily because an increase of the threshold may occur. -
Page 11: Patient Safety
MODEL 3077 – User’s Manual 7 Patient Safety The external pulse generator MODEL 3077 meets all applicable international standards for patient safety: IEC 60601-1 Medical electrical equipment. General requirements for safety IEC 60601-1-2 Medical electrical equipment. Electromagnetic compatibility IEC 60601-2-31 Medical electrical equipment. -
Page 12: Electromagnetic Compatibility
Portable and mobile RF communications equipment, such as mobile phones, can affect the function of MODEL 3077. Mobile phones with a rated maximum output power of 2 Watt and a transmitter frequency up to 2.5 GHz should be used no closer to any part of MODEL 3077 (including patient cables and sensors) than a recommended separation distance of 10 m (30 ft). -
Page 13: Use And Application Of Model 3077
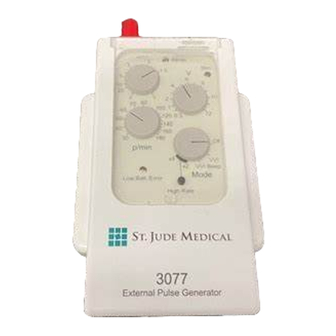
MODEL 3077 – User’s Manual 9 Use and Application of MODEL 3077 9.1 Design MODEL 3077 with its controls and terminals is shown in Figure 1 and Figure 2. Figure 1: External Pulse Generator MODEL 3077 Front Face 5I-17-024X-B-05 13 / 32... - Page 14 MODEL 3077 – User’s Manual Figure 2: External Pulse Generator MODEL 3077 Rear View Lead connection sockets: Protected safety connectors for plugs with a diameter of 0.9 mm to max. 2.0 mm. Different pole (–) : black Indifferent pole (+) : Green LED blinks synchronous to the sensed P-/R-wave.
-
Page 15: Connecting Temporary Stimulation Leads
4’, the stimu- lation rate will be doubled or quadrupled when the “High Rate” button is pushed. MODEL 3077 stimulates at a fixed rate, independent of the set sensitivity value. After the ‘High Rate’ button is released, the pacemaker reverts to the previous mode of operation. -
Page 16: Lead Types
MODEL 3077 – User’s Manual To connect the stimulation leads to MODEL 3077, proceed as follows: MODEL 3077 must be turned off before any lead is connected. Make sure the Mode Switch [5] is in the ‘Off’ position (Figure 1). -
Page 17: Powering On Model 3077
For exact specifications of leads and patient cables, please refer to our product catalog. 9.3 Powering On MODEL 3077 Turn MODEL 3077 on by turning the Mode switch [5] clockwise from the “Off” position to any of the positions ‘VVI’, ‘VVI Beep’, ‘x2’ or ‘x4’. -
Page 18: Modes Of Operation
MODEL 3077 – User’s Manual Warning If the pacemaker is to be used for a longer period of time (for instance, more than 2 hours) on a pa- tient, the cardiac capture threshold should be checked frequently because an increase in the cardiac capture threshold may occur. -
Page 19: High-Rate Stimulation (X2, X4)
9.9 Battery Surveillance MODEL 3077 is powered by a standard 9 V battery. MODEL 3077 continuously monitors the battery voltage. If the red LED (Figure 1 [9]) is blinking, the battery must be replaced. As the battery continues to discharge, the interval of blinking decreases from 5 s to 1 s and an acoustic warning tone is given. -
Page 20: Environmental And Medical Therapy Hazards
MODEL 3077 may be inhibited by strong external interferences that resemble the signal the pacemaker is designed to sense. Such interference may be produced by a variety of sources including electrocautery, diathermy, and other devices. MODEL 3077 will not be damaged by such interference and will resume its function as soon as the interference source is removed. -
Page 21: Storage
For device operation temperature must be in the range of (+10°C (+50°F)…+45°C (+113°F)). Warning In case MODEL 3077 is not used for a longer period of time, remove the battery from the battery compartment in order to prevent damage from possible battery acid leakage. -
Page 22: Care And Maintenance
The enclosure and the keypad of MODEL 3077 are protected against accidental liquid spills. To clean the device, use a towel or sponge moistened with water or alcohol. -
Page 23: Safety Check-Ups Of The Pacemaker
Use 9 V-battery (6LR61) produced only by a reliable battery manufacturers. Do not use rechargeable batteries! 11.3 Safety Check-Ups of the Pacemaker In order to assure safe operation of MODEL 3077, the following check-ups must be carried out on a regular basis. Before each use Visual inspection: ... -
Page 24: Customer Service
MODEL 3077 – User’s Manual 12 Customer Service If you have any questions, the customer service can be reached at: St. Jude Medical USA: 15900 Valley View Court Sylmar, CA 91342 + 1 (818) 362 6822 Europe: + 46 (8) 474 4147... -
Page 25: Technical Data
MODEL 3077: non-sterile Extension cables connecting to pacing electrodes / wires: sterile No protection against inflammable MODEL 3077 must not be used in the presence of inflammable mixtures of anesthesia supplies: anesthesia supplies and air, oxygen or nitrous oxide (N... - Page 26 MODEL 3077 – User’s Manual Parameter Acoustic signaling: Different for stimulation, sensing and warnings; acoustic signaling for stimulation and sensing can be turned on (Mode ‘VVI Beep’) or off (Mode ‘VVI”). Safety Interference recognition: Interference frequencies >283 ppm ± 5 % cause a switch to asynchronous safety...
-
Page 27: Delivery Unit
Arm Strap (L = 45 cm / 17.7”) MODEL 3077 User’s Manual Contact your local St. Jude Medical representative for temporary stimulation electrodes / pacing wires and accessories such as extension cables and adaptors. 5I-17-024X-B-05 27 / 32... -
Page 28: Conformity According To Iec 60601-1-2
Manufacturer guidelines and declarations: Electromagnetic Radiation Standard: IEC 60601-1-2: Table 1 MODEL 3077 is intended for use in an electromagnetic environment as described below. The user should make sure that MODEL 3077 is used in such an environment. Emissions Test... - Page 29 Electromagnetic Immunity for External Cardiac Pacemakers Standard: IEC 60601-1-2: Table 3 MODEL 3077 is intended for use in an electromagnetic environment as described below. The user should make sure that MODEL 3077 is used in such an environment. Testing the Immunity...
- Page 30 Standard: IEC 60601-1-2: Table 5 MODEL 3077 is intended for use in an electromagnetic environment, in which the RF interference is under control. There user of the MODEL 3077 can help to prevent electromagnetic interference by maintaining a safety distance to mobile RF communication equipment (transmitters) –...
-
Page 31: Declaration Of Conformity
MODEL 3077 – User’s Manual 16 Declaration of Conformity Osypka Medical GmbH Albert-Einstein-Strasse 3 12489 Berlin Germany declare under our sole responsibility, that the medical device MODEL 3077 Single-Chamber External Pulse Generator including Accessories is in conformity with the act on Medical Devices, the Medical Device Directive 93/42/EEC amended by 2007/47/EC with reference to Directive 2006/42/EC on Machinery and the applicable medical device standards IEC 60601 - 1 and IEC 60601-2-31. - Page 32 MODEL 3077 – User’s Manual Manufacturer: Distributor: USA: 7855 Ivanhoe Avenue, Suite 226 USA: St. Jude Medical La Jolla, CA 92037, USA 15900 Valley View Court Phone: +1 858 454 0021 Sylmar, CA 91432, USA Fax: +1 858 454 0064 Phone: +1 818 362 6822 E-mail: mail@osypkamed.com...














Need help?
Do you have a question about the 3077 and is the answer not in the manual?
Questions and answers