Advertisement
Quick Links
Advertisement

Summary of Contents for St. Jude Medical Angio-Seal Evolution
- Page 1 INSTRUCTIONS FOR USE Angio-Seal™ Evolution™ Vascular Closure Device...
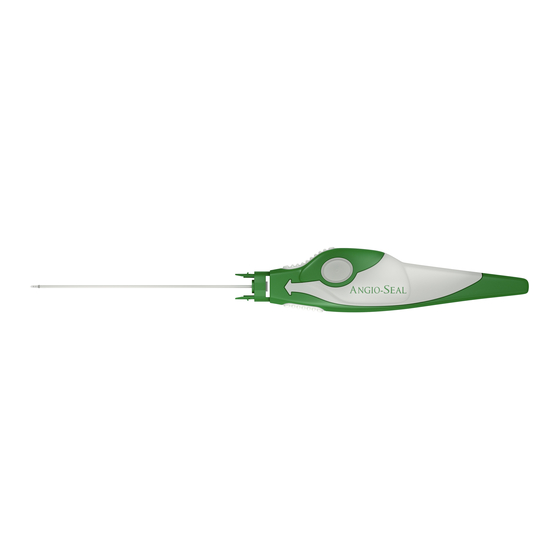
- Page 2 The Angio-Seal™ Evolution™ Vascular Closure Device consists of the Angio-Seal Evolution Device, an insertion sheath, an arteriotomy locator (modifi ed dilator) and a guidewire. The Angio-Seal Evolution Device is composed of an absorbable collagen sponge and a specially designed absorbable polymer anchor that are connected by an absorbable self- tightening suture (STS).
-
Page 3: Adverse Events
ADVERSE EVENTS The Angio-Seal Device was evaluated in a non-randomized clinical trial involving 306 patients in whom the access sites were closed with the device following diagnostic angiography (n=97) or percutaneous coronary intervention (n=209) procedures. Table 1 reports the adverse events as a percentage of patients who received the Angio-Seal Device in the clinical investigation. - Page 4 The effectiveness of the Angio-Seal Device was evaluated by times to hemostasis and ambulation. Time to hemostasis was defi ned as the elapsed time from device deployment until cessation of bleeding. Time to ambulation was defi ned as the elapsed time from device deployment to the time the patient walked for fi ve minutes or 100 feet. Major and overall complication rates comprised the safety endpoints.
-
Page 5: How Supplied
Results of the clinical study demonstrate that patients that have undergone diagnostic angiography and have received a 6F Angio-Seal Device can safely and effectively ambulate in less than 20 minutes and be discharged one hour post ambulation. HOW SUPPLIED The Angio-Seal Evolution Vascular Closure Device is supplied sterile in a bag. This bag contains the following supplies. 1 each: •... - Page 6 Figure 2 Insertion Sheath NOTE: When resistance is encountered at the anterior vessel wall during over-the -guidewire advancement of the Angio-Seal locator/insertion sheath assembly, rotate the assembly 90 degrees so the reference indicator is facing away from the user. This places the beveled tip of the insertion sheath perpendicular to the anterior vessel wall.
- Page 7 b) if necessary, rotate the insertion sheath so that the reference indicator (arrow) on the insertion sheath cap is facing up (Figure 5). Figure 5 Insertion Sheath Patient WARNING: Under normal conditions, the Angio-Seal insertion sheath should not move into or out of the artery for the remainder of the Angio-Seal Device deployment procedure.
- Page 8 3. With one hand, continue to hold the insertion sheath cap steady to prevent movement of the sheath into or out of the artery. With the other hand, grasp the device handle and slowly and carefully pull back. Slight resistance will be felt when the device sleeve is pulled out from the rear holding position. Continue pulling on the device handle until resistance from the anchor catching on the distal tip of the insertion sheath is felt.
- Page 9 C. Seal the Puncture 1. Once the anchor has been deployed correctly (Figure 9), and the device handle has been locked into the full rear locked position (Figure 10), avoid applying pressure to the puncture site with the non-deploying hand. Carefully withdraw the device/sheath assembly along the angle of the puncture tract to position the anchor against the vessel wall (Figure 12). NOTE: Do not attempt to re-insert the device.
-
Page 10: Limited Warranty And Disclaimer
LIMITED WARRANTY AND DISCLAIMER St. Jude Medical (SJM) hereby warrants that if any SJM product fails to perform within normal tolerances for a patient due to a defect in materials or workmanship, SJM will provide, at no charge, a replacement SJM product for the patient’s use. This limited warranty applies only if each of the following conditions is met: 1. - Page 11 SYMBOLS For Single Use Only See Instructions for Use Use By Keep Dry Keep away from sunlight, including UV Store only at temperatures between light. 15°C and 25°C. STERILE R Do not use if temperature indicator dot on package has turned from light grey to dark grey or black.
- Page 12 ART100041600 Ver. A 04/2011 Angio-Seal, Evolution, ST. JUDE MEDICAL, the nine-squares symbol and MORE CONTROL. LESS RISK. are trademarks and service marks of St. Jude Medical, Inc. and its related companies. © 2011 St. Jude Medical. All rights reserved.












Need help?
Do you have a question about the Angio-Seal Evolution and is the answer not in the manual?
Questions and answers