Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for natus Algo 7i
- Page 1 User Manual...
- Page 2 Page 2 / 40...
- Page 3 This Manual is applicable to the following Part Numbers: A7i-101049 All mentioned items, products, brands and trademarks are registered or owned by the mentioned companies. AABR, ALGO, Flexicoupler, Jelly Tab, and Natus are registered trademarks of Natus Medical Incorporated. PATH MEDICAL GmbH Landsberger Straße 65...
-
Page 4: Table Of Contents
Table of Contents Overview ............................7 Introduction ..........................7 Intended Use ........................... 7 1.2.1 Intended Purpose ......................7 1.2.2 Contraindications ......................8 1.2.3 Exclusion Criteria ......................8 1.2.4 Side Effects ........................8 Performance Characteristics ....................8 Explanation of Symbols ......................10 Basic Operation ......................... - Page 5 Repair ............................ 26 Cleaning ............................. 27 Accessories ..........................28 Warranty............................ 29 Notes on Safety ......................... 31 General Usage ........................31 Handling, Transport, and Storage..................31 Electrical Safety ........................32 Electromagnetic Compatibility ....................33 Accessories ..........................33 Waste Disposal ........................34 Technical Specifications ......................
-
Page 7: Overview
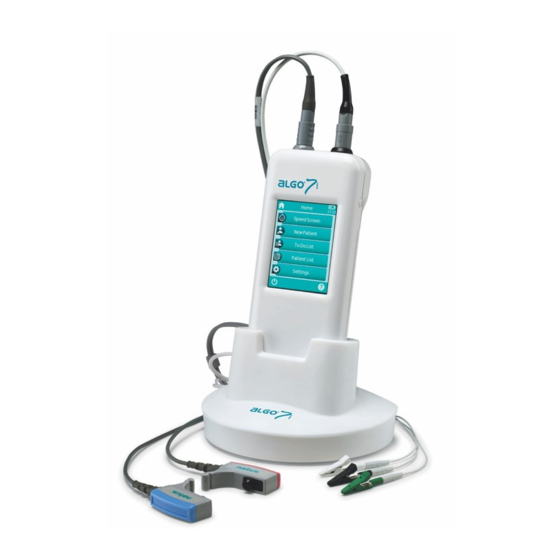
1 Overview 1.1 Introduction Thank you for purchasing the ALGO 7i. This manual is your guide for safely operating and maintaining your device. Please read this manual carefully before using the ALGO 7i the first time. We recommend taking particular note of the safety (see section 8: Notes on Safety), intended use (see section 1.2: Intended Use), cleaning (see section 5: Cleaning) and maintenance (see section 4: Service... -
Page 8: Contraindications
The ALGO 7i is not intended for operational use by the general public. All test procedures must be supervised or conducted by qualified personnel. In the United States of America, Federal law restricts this device to sale by or on the order of a licensed physician. - Page 9 The ALGO 7i hearing screener offers the user the option to screen both ears simultaneously, both ears sequentially or each ear individually. This single technology device uses Natus’s patented AABR® (Automated Auditory Brainstem Response) Screening technology to determine the status of the hearing pathway from the outer ear up to the brain stem.
-
Page 10: Explanation Of Symbols
2 Explanation of Symbols This section explains all symbols used within this manual and on the device label. Symbols within this manual: Symbol Explanation Important notice: please read for important information. Warning: please read for safety-relevant information, which may cause risk of danger to persons and/or device if not followed. -
Page 11: Basic Operation
3 Basic Operation After switching on the device, the device can be operated via a touch-sensitive display. In the following section, the most important device functions and screen elements are explained. Please note that screen shots or references to test modules in this manual may not reflect the actual configuration of your device. -
Page 12: Online Help
3.2 Online Help Content-sensitive help screens allow intuitive handling of the device. Automatically generated message boxes may present additional content-sensitive warnings or information. The content-sensitive help screens are available via the question mark icon, which is displayed in the footer. The help screens explain the currently available symbols and their functions. 3.3 Test Result Status Icons In the test history list, test results are shown with an overall test result status icon. -
Page 13: Device Reset
Alternatively, the device can be switched off via the off-switch icon in the footer of the device display. 3.4.2 Device Reset If the touch screen is unresponsive (i.e., no reaction when pressing an icon), reset the device by pressing the on/off switch for several seconds. The reset does not change any device or test module settings or affect any other saved data on the device. -
Page 14: Charging The Device
Please make sure that local data protection requirements are met. When user management is deactivated on ALGO 7i device, the device does not provide any inherent access protection (i.e. no login with password). Page 14/40... -
Page 15: Patient Management
3.5.2 Patient Management After switching on the device (and if applicable after login) a patient can be added, selected from the list of patients or the test screen can be entered directly in “Speed Screen” mode, i.e. without adding or choosing a patient. Depending on user rights, it is also possible to delete a single patient, all patients or patients by date range (Device Settings →... - Page 16 Following information then can be entered using the keyboard on the touch display: When done, confirm by tapping the check mark. You’ll be automatically asked if you want the patient added to the To-Do List. Once a Patient has been saved to the Patient and/or To-Do list, following options are available: “Start Screen”, “Select Protocol”...
- Page 17 When adding Patient to the To-Do- List, you can create your individual work list for each day. Either load your To-Do- list using the ALGOLink PC Software onto your device, or choose from the patient database on your ALGO 7i device and tap on the “add patient” button in the footer of the Patient Details screen.
-
Page 18: Device Settings
① Print Record, ② Remove Patient from To-Do List). related options in the Footer ( When Removing a Patient, please choose the reason for removal. ① ② 3.5.3 Device Settings There are multiple options to configure the device to your needs. The device settings can be reached via Settings Tab with the gearwheel symbol on the main screen: The following device settings are available: Device Management, date and time format... -
Page 19: Hardware Tests
7i Check Kit to test the Impedance levels with your cable. To do so, plug in your Patient Cable in the black socket of your ALGO 7i device and attach all three clips to the metal bar of your Check Kit. -
Page 20: System Information
If the recommended actions in Table 3 do not help in solving the problem, please contact Natus Technical Service or your Natus authorized service representative. 3.5.5 System Information On the system information screen, general information about the device and firmware version is displayed. Information about connected transducers is also displayed if the respective transducer has been connected before the system information screen is entered. - Page 21 Carefully align the ridges on the plug to the slots in the port to reduce the chance of bending the metal pins inside the plug. You must insert the plugs completely into the corresponding ports for the device to operate properly. Never twist the plugs during insertion or removal. Twisting a plug in the port can damage the metal pins and cause system malfunctions.
- Page 22 New Patient button or by selecting a patient from the To-Do List. The screening options appearing on your device are set by your program administrator. The ALGO 7i device supports simultaneous, sequential, or single screening of each ear individually.
-
Page 23: Troubleshooting
Start screening: Select the Patient to be screened: • To select a Patient already added to the To-Do list, on the Home screen, tap To-Do List and select the patient, and select start screen, or select protocol, and start screen depending on your device settings •... -
Page 24: Algolink Pc Software
PC printer. The ALGOLink Software comes with a built-in online help for further information about correct usage and for troubleshooting. This software also includes the latest firmware for updating the ALGO 7i device. Please contact Natus Technical Service or your Natus authorized service representative to obtain the latest version of this software. -
Page 25: Service And Maintenance
System Info in the Device Settings Tab. Note: The ATA calibration date is not related to the current time and date values in the ALGO 7i screener. Changing the current time and date will not affect the calibration clock. -
Page 26: Repair
4.3 Repair If a defect of any kind is suspected, or calibration of cables is due, Natus, or an authorized service partner will repair, re-calibrate or exchange the device or accessory. All repairs are subject to parts and material availability. -
Page 27: Cleaning
5 Cleaning Cleaning the device and its accessories is very important for compliance with hygienic requirements and to avoid any cross-infection. Please always consider local regulations and read this section carefully. Before cleaning the device, the device must be switched off and removed from all connected components (e.g. -
Page 28: Accessories
6 Accessories Available accessories for ALGO 7i devices include: Type Model examples Applied part Max. cable length* ATA Cable 1.83 m (72’’) or 0.91m (36”) Related accessories: Flexicoupler Patient Cable Patient Cable 1.83 m (72’’) or 0.91m (36”) Related accessories:... -
Page 29: Warranty
Natus Medical reserves the right to credit, repair or replace (with a new or refurbished product) an “in-warranty” device or accessory. Warranty repairs for the ALGO 7i are handled in the same manner as other repairs and service. See also section 4.1: General Service Information. -
Page 31: Notes On Safety
8 Notes on Safety In order to allow safe performance of the ALGO 7i, please read the following notes on safety carefully and follow the provided instructions. If not followed, risks of danger to persons and/or the device may be the consequence. Retain this manual for later use and make sure to hand over this manual to any person who uses this device. -
Page 32: Electrical Safety
9: Technical Specifications. The use of alternate power supplies designed for other electronic devices such as notebook computers or printers may cause damage to the device. Likewise, using the ALGO 7i power supply on other types of devices may cause damage to those devices. -
Page 33: Electromagnetic Compatibility
8.4 Electromagnetic Compatibility The use of ALGO 7i devices in close proximity to other electronic equipment or with other electronic equipment in a stacked form should be avoided, as this could result in improper operation (e.g. occurrence of unwanted noise). Electronic equipment may include e.g. -
Page 34: Waste Disposal
Keep small parts out of patient’s range in order to prevent accidental swallowing. No parts may be eaten, burnt, or in any other way used for purposes other than specified in the intended use. The sockets are intended to connect to the respective accessories (ATA Cable, Patient Cable, Multi-Data Cable). -
Page 35: Technical Specifications
Typical power consumption from ca. 12 V, 0.17 A = 2 W power supply unit during charging 9.3 Power Supply For medical applications the following power supply units are exclusively allowed when used with ALGO 7i devices: • Friwo FW7662M/12 Page 35/40... - Page 36 TRANSPORT AND STORAGE CONDITIONS: Transport temperature -20 to 60 °C (-4 to 140 °F) Storage temperature 0 to 40 °C (32 to 104 °F) Relative air humidity 10 to 90 % non-condensing Barometric pressure 50 to 106 kPa OPERATING CONDITIONS: Temperature 10 to 40 °C (50 to 104 °F) Relative air humidity...
-
Page 38: Electromagnetic Compatibility Information
10 Electromagnetic Compatibility Information Electromagnetic compatibility (EMC) as stated by standard DIN EN 60601-1-2 (Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic and 60601-2-40 compatibility - Requirements and tests) (Medical electrical equipment - Part 2-40: Particular requirements was certified by an accredited laboratory. - Page 39 Tests for immunity IEC 60601 test level Concurrent level Electromagnetic environment to interference Voltage drop, short 0 % U (>95 % U 0 % U (>95 % U The quality of supply voltage interruption and drop) for ½ and 1 drop) for ½* and 1 shall correspond to typical fluctuation of supply...
- Page 40 Contact information from distributor/service partner: Distributed By: Natus Medical Incorporated 3150 Pleasant View Road, Middleton, WI 53562 USA Toll-free: +1-800-303-0306 Telephone: +1-650-802-0400 Fax: +1-650-802-8680 E-mail: technical_service@natus.com www.natus.com PATH MEDICAL GmbH Landsberger Straße 65 82110 Germering Germany Page 40 / 40...















Need help?
Do you have a question about the Algo 7i and is the answer not in the manual?
Questions and answers