Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for natus Dantec Clavis
- Page 1 ™ Dantec Clavis ® User Guide 9031M6114EN rev.B...
- Page 2 Dantec Clavis User Guide Blank page.
- Page 3 Guide Copyright © 2018 Natus. All rights reserved. The contents of this manual are the property of Natus Medical Incorporated. Any reproduction in whole or in part is strictly prohibited. At the time of printing / transfer to the CD-ROM, this manual correctly described the device and its functions.
- Page 4 Dantec Clavis User Guide Blank page.
-
Page 5: Table Of Contents
Declaration of Compliance for FCC ......................30 Figures: Figure 1. Control Panel Overview ......................12 Figure 2. EMG Electrode Connections ....................14 Figure 3. Electrode Stimulation Connections ..................16 Figure 4. Dantec Clavis rear side, the battery compartment..............20... - Page 6 Dantec Clavis User Guide Blank page.
-
Page 7: Description Of Symbols
Attention: Please read the instruction manual before using this device. The Dantec Clavis system is certified to carry the CE mark. The CE mark is a declaration that this product is in compliance with the directive set forth by the European Union for medical devices. - Page 8 Dantec Clavis User Guide Medical device listing mark for U.S. and Canada by Intertek Testing Service. Follow instructions for use. The device is not user serviceable. Battery type.
-
Page 9: Safety Information
Do not use damaged or defective devices. Protect this instrument from immersion, spills, the impact of falling objects, and exposure to excessive smoke, dust, mechanical vibration, or shock. Intended Use Dantec Clavis is a medical device intended as a stimulator for nerve localization as well as an aid for guidance of injections into the muscles. -
Page 10: Essential Performance
Guide Essential Performance Essential performances of the Dantec Clavis product are identified in the standard IEC 60601-2-40, Edi- tion 2.0, 2016-08, Particular requirements for the basic safety and essential performance of electromy- ographs and evoked response equipment. Essential performance relates to the quality of the signal recorded from the amplifier. -
Page 11: Side Effects
2. Over the left and/or right temporal regions 3. In the orbital region Use in an anesthetic environment: The Dantec Clavis system is not suitable for use in presence of a FLAMMABLE ANAESTHETIC MIXTURE WITH AIR or WITH OXYGEN or NITROUS OXIDE. ... -
Page 12: Operating Dantec Clavis
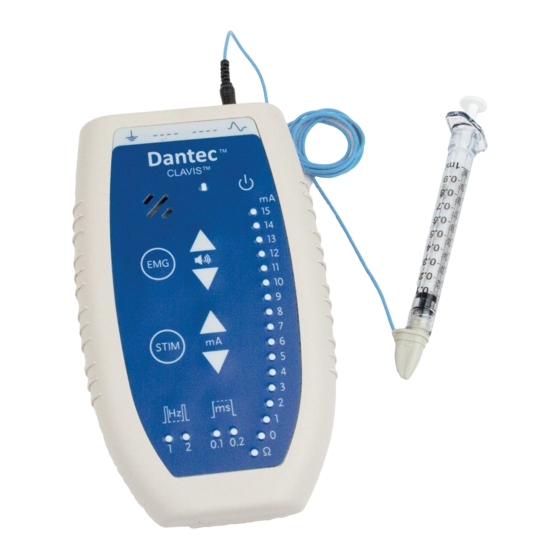
Dantec Clavis User Guide Operating Dantec Clavis Control Panel Overview Dantec Clavis Front Panel Button/ Symbol Legend Patient Ground. Reference. EMG input. Speaker Battery power status indicator– yellow light. Power switch (on/off toggle). Activation button. Volume controls. Stimulation Activation button. -
Page 13: Start - Autotest
Press the Power button again to switch off the device. When you switch on Dantec CLAVIS, it will start an internal auto test, while at the same time it will allow you to verify that the Sound and Indicator Light functions are working properly. -
Page 14: Emg Mode
In the EMG mode, the device is used for needle electrode examinations. Throughout the procedure, Dantec Clavis will emit a series of audible signals varying in intensity and frequency that will help moni- toring the localization of the targeted muscle or nerve. -
Page 15: Emg Buttons
Dantec Clavis User Guide Attaching the Electrodes to the Patient Once the electrode leads have been connected to the device, you can attach the ground and reference electrodes to the patient, and when ready, proceed with the EMG needle electrode (active input). -
Page 16: Stimulation Mode
Electrodes Connecting the Electrode Leads to the Device CAUTION: Only electrodes recommended by Natus must be used. Refer to the Neurodiagnostic Supplies section of this user guide for further information. Before starting the procedure, connect the surface and needle electrode leads to their corresponding color-coded connectors as shown in fig. -
Page 17: Stimulation Buttons
Dantec Clavis User Guide Attaching the Electrodes to the Patient Once the electrode leads have been connected to the device, you can attach the surface electrode/s (anode) to the patient, and when ready, proceed with the needle electrode (cathode). WARNING: Avoid trans-thoracic stimulation. Keep the anode and cathode stimulation sites in close proximity. - Page 18 Dantec Clavis User Guide Stimulation Level Bar –The Stimulation Level bar ranges from 0mA to 15mA. –The Stimulation Level indicators show the level of current selected. –When Stimulation mode is activated, the indicators are flashing. Overload Indicator –When the Overload indicator is lit up, it indicates that the device is unable to deliver the selected current.
-
Page 19: Cleaning
Dantec Clavis User Guide Maintenance Dantec Clavis requires no user maintenance other than cleaning the device after each use and replacing the battery periodically. Cleaning The cleaning procedure must be in accordance with your local hygiene authority’s guidelines. Before you start cleaning the device, make sure it is switched off and that the electrode leads are dis- connected. -
Page 20: Waste Management
Dantec Clavis User Guide Access to the Battery Compartment Figure 4. Dantec Clavis rear side, the battery compartment. Turn off the device. Slide open the battery compartment lid on the rear of the device. Remove the old battery by pulling it up from the bottom end. -
Page 21: Neurodiagnostic Supplies
Dantec Clavis User Guide Neurodiagnostic Supplies Natus recommends the use of the following accessories electrodes and cables with Dantec Clavis. ® Bo-ject DHN Disposable Hypodermic Needle Electrodes ® Bo-ject DHN Disposable Hypodermic Needle Electrode: Needle Part Number Needle Length Lead Color... -
Page 22: Technical Data
Dantec Clavis User Guide Technical Data Power Supply Power supply: internally powered equipment: one 9V alkaline battery. IEC-6LR61, ANSI-1604A. Power consumption: maximum 2 watts. Weight 185 g. with battery (6.526 Oz) 140 g. without battery (4.938 Oz) Dimension (L x W x H) ... - Page 23 Dantec Clavis User Guide STIM Mode Output current: 1.0 – 15.0mA. Adjustable in steps of 1mA. Electrode impedance: 200 – 7 kΩ. Maximum excitation voltage: 100V When impedance is higher than 7 kΩ, the device cannot deliver full current stimulus. The Overload indicator will be activated if the selected current level cannot be delivered.
-
Page 24: Safety & Standards Conformity
Safety & Standards Conformity Standards of Compliance and Normative References The Dantec Clavis System, powered by a 9V battery with the following levels of protection 1. Type of protection against electric shock: Class II 2. Degree of protection against electric shock: Type BF 3. - Page 25 Dantec Clavis User Guide Table 2 – EMC Standard of Compliance and Normative References IEC 60601-1-2, Edition 4.0, February 1, 2014 Medical electrical equipment – Part 1-2: General re- quirements for basic safety and essential perfor- mance– collateral standard: electromagnetic com- patibility –...
-
Page 26: Declaration Of Compliance For Iec 60601-1-2, 4Th Edition
Guidance and manufacturer’s declaration – electromagnetic emissions The Dantec Clavis is intended for use in the electromagnetic environment specified below. The cus- tomer or the user of the Dantec Clavis should assure that it is used in such an environment. Emissions Test... - Page 27 Guidance and manufacturer’s declaration – electromagnetic immunity The Dantec Clavis is intended for use in the electromagnetic environment specified below. The cus- tomer or the user of the Dantec Clavis should assure that it is used in such an environment. Immunity Test...
- Page 28 To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which Dantec Clavis is used ex- ceeds the applicable RF compliance level above, Dantec Clavis should be observed to verify normal operation. If abnormal operation is observed, additional measures may be necessary, such as re-orienting or relocating Dan- tec Clavis.
- Page 29 Dantec Clavis User Guide Table 4 - Test specifications for ENCLOSURE PORT IMMUNITY to RF wireless communications equip- ment Test Band Maximum Distance IMMUNITY Modulation frequency Service Power TEST LEVEL (MHz) (MHz) (V/m) Pulse 380 –390 TETRA 400 modulation 18 Hz GMRS 460, ±...
-
Page 30: Declaration Of Compliance For Fcc
Dantec Clavis User Guide Declaration of Compliance for FCC Note: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable pro- tection against harmful interference when the equipment is operated in a commercial en- vironment. - Page 31 Dantec Clavis User Guide Please consult www.natus.com for your local sales & service office. Natus Manufacturing Limited IDA Business Park Gort, Co.Galway, Ireland Rx only...
- Page 32 Dantec Clavis User Guide Blank page.
















Need help?
Do you have a question about the Dantec Clavis and is the answer not in the manual?
Questions and answers