Summary of Contents for Bien Air CA 1: 2.5 L MS
- Page 1 CA 1: 2.5 L MS ENG INSTRUCTIONS FOR USE Other languages available on www.bienair.com/ifu REF 2100337-0002/2020.06 Rx Only...
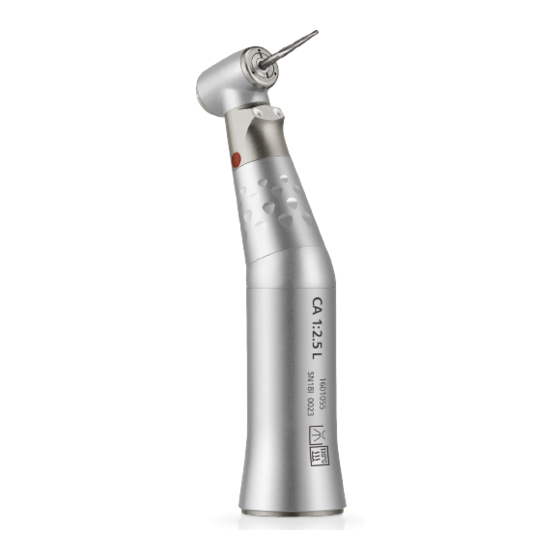
- Page 2 Set supplied (REF) CA 1:2.5 L MS 1601055-001 Optional accessories (REF) 1600617-006 1600036-006 1600064-006 1000001-010 REF 2100337-0002/2020.06 CA 1:2.5 L MS • © Bien-Air Dental SA...
-
Page 3: Table Of Contents
Table of contents 1 Symbols 7 Transport& disposal 1.1 Description of symbols used 7.1 Transport and storage con- ditions 2 Identification & Intended Use 5 7.2 Disposal 2.1 Identification 8 General information 2.2 Classification 2.3 Intended use 8.1 Terms of guarantee 8.2 References 3 Precautions for use 8.2.1 Set supplied (see cover) -
Page 4: Symbols
ENG INSTRUCTIONS FOR USE 1 Symbols Description of symbols used Description Description Manufacturer. Reference number. CE Marking with number of the notified Serial number. body. WARNING:hazard that could result in ser- ious injury or damage to the device if the Medical Device. -
Page 5: Identification & Intended Use
2 Identification & Intended Use 2.1 Identification 2.3 Intended use Medical devices manufactured Product intended for professional use Switzerland by Bien-Air Dental SA. only. Used in general dentistry and oral surgery. Type Contra-angle handpiece (CA) speed mul- tiplier ratio with light and external WARNING irrigation. -
Page 6: Precautions For Use
3 Precautions for use Those medical devices must be used by CAUTION a competent person, in particular in It is essential to use dry, purified com- compliance with the legal provisions in pressed air in order to ensure the long force regarding occupational safety, working life of the device. -
Page 7: Description
FIG. 1 (CA 1:2.5 L MS) 4 Description 4.1 Overview FIG. 1 (1) Centering for the micromotor (2) Light output (3) Bur (not supplied) (4) Transmission ratio marking clip (5) Push-button 4.2 Technical data Contra-angle 1:2.5 Standard coupling As per ISO standard 3964* Drive speed 40’000 rpm max. - Page 8 FIG. 2 FIG. 3 Bur chuck FIG. 2 Shaft diameter 1.60 mm, type 3 as per ISO 1797-1, recommended length 21 mm* code 4/5 as per ISO 6360-1 for the 1:2.5 range (Maximum working dia- meter 2 mm). (*) When using longer rotary in- struments (e.g.
-
Page 9: Operation
5 Operation WARNING The use of a coolant while the in- 5.1 Changing the bur strument is in operation is mandatory, failure comply with this FIG. 3 commendation result Push-button bur locking. overheating and burns or product fail- 1. Press the push-button and sim- ure. -
Page 10: Cleaning And Servicing
6 Cleaning and CAUTION servicing Carry out cleaning-disinfection- sterilisation without a bur in the chuck mechanism. 6.1 Maintenance - General in- Do not immerse in an ultrasonic formation cleaner. Do not submerge in physiological Clean, lubricate and sterilise the device liquid (NaCI). -
Page 11: Cleaning
FIG. 4 FIG. 5 6.2 Cleaning Preparation 1. Disconnect the device from the elec- trical motor and remove the bur (FIG. 4 step 1). Remove dirt / deposits FIG. 4 step 1 and 2 1. Clean under tap water at 15°C-38°C. 2. Carefully remove all traces of dirt or deposits from the nozzles using the Bien-Air cleaning wire (optional). - Page 12 FIG. 6 FIG. 7 Internal and external cleaning 1. After selecting the appropriate end- piece, spray the inside and the out- side of the device with the product Spraynet (FIG. 6 and FIG. 7). 2. Dry with non-woven compresses.
-
Page 13: Disinfection
FIG. 8 6.3 Disinfection with Spraynet and drying it with sterile non-woven compresses. 5. After selecting the appropriate 6.3.1 Manual cleaning / disinfection nozzle, spray inside the device with 1. Dip the device in a bath containing a Spraynet (FIG. 8). disinfectant product (e.g. di- decyldimethylammonium chloride, 6. - Page 14 Recommended specifications for the thermo-disinfection cycle. Phase Parameters Pre-cleaning <45°C (113°F); ≥ 2 minutes Cleaning 55°C-65°C (131°F-149°F); ≥ 5 minutes Neutralization ≥ 2 minutes Rinsing Tap water, ≤30°C (86°F), ≥ 2 minutes cold water Thermal Disinfection Demineralized water, 90°C-95°C (194°F-203°F), 5-10 minutes Drying 18-22 minutes CAUTION...
-
Page 15: Lubrication
FIG. 9 6.4 Lubrication Procedure 6.4.1 Verifying cleanliness CAUTION Visually inspect the device to ensure it is Pack the device in a packaging ap- clean. Repeat the cleaning and dis- proved for steam sterilisation. infection procedure if necessary. CAUTION 6.4.2 Lubrication with Lubrifluid Only use dynamic air removal cycles: FIG. 9 pre-vacuum or steam flush pressure... -
Page 16: Packing
7 Transport 6.6 Packing The device must be stored inside the & disposal sterilisation pouch in a dry and dust- free environment. The temperature 7.1 Transport and storage must not exceed 55°C (131°F). If the conditions device will not be used for 7 days or more after the sterilisation, extract the Follow the general requirements ap- device from the sterilisation pouch and... -
Page 17: General Information
8 General 8.2 References information 8.2.1 Set supplied (see cover) Legend 8.1 Terms of guarantee 1601055-001 CA 1:2.5 L MS contra-angle Bien-Air Dental SA grants the user a warranty covering any operating fault, 8.2.2 Optional accessories or material or manufacturing defect. (see cover) The warranty period for this medical device is 12 months from the date of in-... - Page 18 NOTES...
- Page 19 NOTES...
- Page 20 Bien-Air Dental SA Länggasse 60 Case postale 2500 Bienne 6 Switzerland Tel. +41 (0)32 344 64 64 Fax +41 (0)32 344 64 91 dental@bienair.com Other adresses available at www.bienair.com REF 2100337-0002/2020.06 CA 1:2.5 L MS • © Bien-Air Dental SA...















Need help?
Do you have a question about the CA 1: 2.5 L MS and is the answer not in the manual?
Questions and answers