Table of Contents

Summary of Contents for Chattanooga I-Bresis Patch
- Page 1 Patch (5000060) - Instructions For Use I-Bresis™ Patch Carton Contents: • 6 Patches • 6 Saline Ampoules • 6 Alcohol Preps READ THE I-BRESIS CONTROLLER AND CHARGING STATION ™ INSTRUCTIONS FOR USE FOR ADDITIONAL IMPORTANT INFORMATION.
- Page 2 GLOSSARY OF SYMBOLS This device may contain one or more of the following symbols: Consult Instructions Keep Away from Sunlight For Prescription Only Caution: Federal law (USA) restricts this device to ONLY Medical Equipment Classified by Underwriters Laboratories Inc., sale by or on the order of a physician. with respect to electric shock, fire and mechanical hazards only in No.
- Page 3 INDICATIONS The I-Bresis™ System is indicated for the administration of soluble salts or other drugs into the body for medical purposes as an alternative to hypodermic injection. FOR ADDITIONAL IMPORTANT INFORMATION, READ THE I-Bresis™ CONTROLLER AND CHARGING STATION INSTRUCTIONS FOR USE. CONTRAINDICATIONS Implanted Electronic Devices •...
- Page 4 WARNINGS Children • – Keep out of the reach of children. Across Chest • – Do not apply stimulation across the patient’s chest, because the introduction of electrical current into the chest may cause rhythm disturbances to the patient’s heart, which could be lethal. Across Head •...
- Page 5 PRECAUTIONS • Drug Information – Consult directions for the use of the drug/compound before delivery. Some drugs/compounds require a specific polarization for use. Observe the indications, contraindications, warnings and precautions related to this issue. Single Use – Do not use electrodes that have been previously used. These patches have been designed for single use only. •...
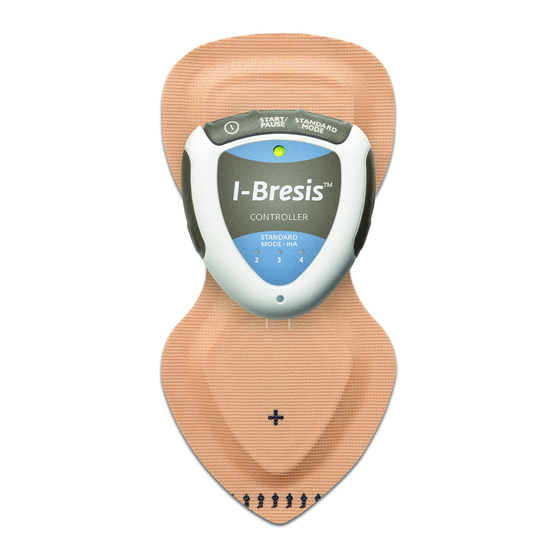
- Page 6 PATCH DESCRIPTION AND INTENDED USE The I-Bresis™ Patch is a disposable, single-use Patch with an internal battery and current-limiting circuitry. It can deliver both negatively and positively charged water-soluble drugs/compounds across intact skin. The Patch can be used with and without the Controller. The Controller is used with the Patch to deliver an I-Bresis™...
- Page 7 THEORY OF OPERATION The I-Bresis™ Iontophoresis System operates on the physical principle that electrically like-charges repel each other. A positively charged substance is forced away from a positively charged electrode. A negatively charged substance is forced away from a negatively charged electrode. When the Patch pads are filled with ionic saline or drug solutions and the Patch is attached to the patient’s skin, current begins flowing.
- Page 8 PREPARING THE PATCH Tear open the sealed treatment kit and remove the Patch. Place the Patch on a flat surface with the absorbent pads facing up. Clean the treatment site thoroughly with alcohol prep by rubbing for six to eight seconds to remove dry skin, oils and other contaminants.
- Page 9 Remove the adhesive release liner from the hydrated Patch. Apply the hydrated Patch so that the drug pad is over the treatment site and secure it by pressing the adhesive border. Avoid pressing directly over the pads. Pressing directly on the pads can cause leakage that will compromise adhesion to the patient. NOTE: DO NOT tape or bind the Patch during treatment.
- Page 10 The average time to complete the dose is indicated in the following table. To prevent excessive dosing, the Patch automatically switches off iontophoresis after the maximum dose has been administered. I-Bresis™ Mode 40 mA-minutes 60 mA-minutes 80 mA-minutes Wear Time 1 hour 1.5 hours 2 hours...
- Page 11 PATCH-ONLY TREATMENT Review the “Instructions for Home Use” at the end of this document with the patient, and provide the patient with a copy of those instructions, if requested. If the patient will be applying additional Patches at home, provide the patient with a copy of this instruction booklet.
- Page 12 EXPECTED LIFE AND DISPOSAL • The Best If Used By date for the Patch is shown on the Patch package and carton. The Patches may not be effective if they are used past the Best If Used By date. • The Patch is designed for only one use.
- Page 13 Removing the Patch: Remove the Patch after the time determined by your clinician. Do not wear the Patch longer than four hours. Wearing the Patch longer increases the risk of skin irritation. After removing the Patch, rinse the treatment area and pat dry with a clean cloth. The Patch is designed for only one use.
- Page 14 PATCH SPECIFICATIONS Classifications: Internally Powered. Type BF Applied Part Battery: Contains two 3-Volt non-rechargeable lithium manganese dioxide button cell batteries Environmental Conditions: Storage: 15°C - 30°C (59°F- 86°F) DO NOT expose to temperatures above 50°C (122°F) Flammability: Do not use around flammable gasses, liquids or materials Treatment Modes: I-Bresis™, Standard, and Patch Only Dimensions:...
- Page 15 DJO Global, LLC 1430 Decision St. Vista, CA 92081 USA 800.336.6569 760.727.1280 14-73101 Rev. B © 2017 DJO, LLC, Inc. DJOglobal.com...
















Need help?
Do you have a question about the I-Bresis Patch and is the answer not in the manual?
Questions and answers