Table of Contents
Advertisement
Advertisement
Table of Contents
Troubleshooting

Summary of Contents for Cardioline HD+
- Page 1 User manual Rev. 14 – 23.04.2019 1936...
- Page 2 All rights reserved © Cardioline SpA. CARDIOLINE® is a registered trademark of Cardioline SpA. This publication may not be reproduced, in whole or in part, in any form or manner, without prior written authorisation Cardioline Spa Via Linz, 151 38121 Trento (TN)
-
Page 3: Table Of Contents
Summary GENERAL INFORMATION .........................1 1.1. Other important information ......................1 SAFETY INFORMATION ..........................3 ELECTROMAGNETIC COMPATIBILITY (EMC) .....................9 3.1. Guidance and manufacturer's declaration of electromagnetic emissions ........9 3.2. Guidance and manufacturer's declaration on electromagnetic immunity ........9 3.3. Guidance and manufacturer's declaration on electromagnetic immunity ........10 3.4. - Page 4 6.3. Switching on the device ........................23 6.4. Connection to the receiver ......................23 EXECUTION OF THE EXAMINATION ......................25 7.1. Preparing the patient ........................25 7.1.1. Preparing the patient's skin .....................25 7.2. Connecting the patient ........................25 7.3. ECG acquisition ..........................27 MAINTENANCE AND TROUBLESHOOTING ....................29 8.1.
-
Page 5: General Information
Other important information This manual was written with the utmost care. Should you find any details which do not correspond to those contained in this manual, please inform Cardioline SpA who will correct such inconsistencies as soon as possible. The information contained in this manual is subject to change without notice. - Page 6 GENERAL INFORMATION...
-
Page 7: Safety Information
SAFETY INFORMATION SAFETY INFORMATION Cardioline SpA will be held responsible for the safety, reliability and functionality of the devices only if: 1. the assembly operations, modifications or repairs are carried out by Cardioline SpA or by its Authorised Service Centre;... - Page 8 SAFETY INFORMATION This device is designed to be used only with the electrodes specified in this manual. Strictly follow the correct clinical procedures to prepare the skin before the application of the electrodes and monitor the patient in order to avoid any irritation, inflammation or other skin reactions. The electrodes are designed for short-term applications and must be promptly removed once the examination is complete.
- Page 9 SAFETY INFORMATION No risk has been highlighted from even prolonged contact with the material HD+ Safety Shell and HD+ Stress Belt are made of. However, it is recommended to avoid direct contact with the skin for periods exceeding 24 hours. ...
- Page 10 SAFETY INFORMATION The device must be handled with care by taking all the necessary precautions in order to prevent and avoid heat sources, liquids and anything else that may damage it. Notes The movements of the patient may generate excessive noise and affect the quality of the ECG tracing or the correct analysis of the device.
- Page 11 700 mbar; 1060 mbar The HD+ must be connected to the receiver before use. In order to be used with the HD+, the receiver must be pre-set in the factory or meet the hardware specifications set out by Cardioline.
- Page 12 SAFETY INFORMATION...
-
Page 13: Electromagnetic Compatibility (Emc)
See par. 3.4 for the recommended separation distance between the radio equipment and the device. The use of accessories and cables other than those recommended by Cardioline SpA, may cause an increase in emissions or a lowering in the protection of the system. -
Page 14: Guidance And Manufacturer's Declaration On Electromagnetic Immunity
ELECTROMAGNETIC COMPATIBILITY (EMC) IEC 61000-4-2 +/- 8 kV air +/- 8 kV air relative humidity should be at least discharge discharge 30%. +/- 2 kV for power supply Electrical Fast Transient/ lines Burst Not applicable IEC 61000-4-4 input/output lines +/- 1 kV Surges differential mode Not applicable... -
Page 15: Recommended Separation Distances Between Portable And Mobile Rf Communications Equipment And The Device
ELECTROMAGNETIC COMPATIBILITY (EMC) manufacturer and d is the recommended separation distance in metres (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey (a), should be less than the compliance level in each frequency range (b). Interference may occur in the vicinity of equipment marked with the following symbol: a. - Page 16 ELECTROMAGNETIC COMPATIBILITY (EMC) is the maximum output power rating of the transmitter in watts (W) according to the specifications supplied by the manufacturer. NOTE 1: At 800 MHz, the separation distance for the higher frequency range applies. NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
-
Page 17: Symbols And Label
SYMBOLS AND LABEL SYMBOLS AND LABEL 4.1. Explanation of the symbols Symbol Description Read the instruction before using the device Comply with the instructions in the use manual CE marking – compliance with the European Union directives 1936 Year of manufacture Type CF equipment protected against electrical shock Separate collection of electrical waste and electronic equipment RF transmitter... - Page 18 SYMBOLS AND LABEL...
-
Page 19: Introduction
INTRODUCTION INTRODUCTION 5.1. Purpose of the manual The manual represents a guide for the execution of the following operations: Reasonable use of the HD+ acquisition device and its functions. Preparation of the device for use. Execution of an ECG examination. ... -
Page 20: Description Of The Device
The HD+ transmits the acquired data wireless and in real-time to a computer/device (i.e. PC or Tablet) where one of the compatible CARDIOLINE software is installed. HD+ uses a standard Bluetooth data transmission technology to transmit 12-lead ECG data over a proximity range, providing perfect electrical insulation and freedom of movement for the patient. -
Page 21: General Overview
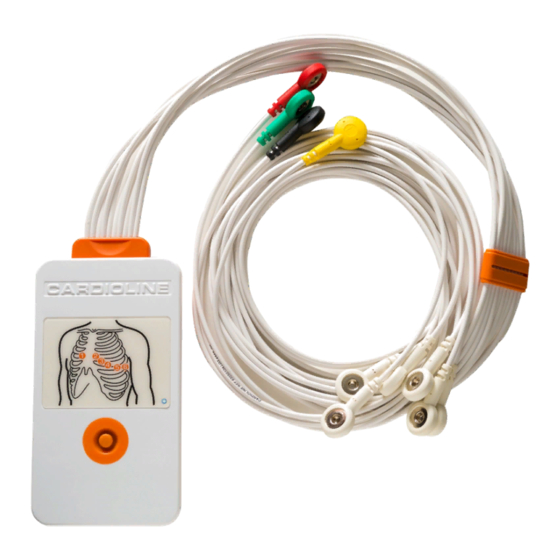
INTRODUCTION 5.5. General overview Front view of the device with patient cable: Patient cable Applied part – CF type Operation LED Power key... -
Page 22: Use Of The Button
INTRODUCTION Rear view of the device: Name label Battery compartment door 5.5.1. Use of the button Use the central button to turn on the device. The device automatically switches off after 5 minutes of inactivity or when the batteries are removed. 5.5.2. -
Page 23: Accessories
INTRODUCTION 5.6. Accessories 5.6.1. HD+ Safety Shell HD+ Safety Shell is a silicone protective shell which allows the HD+ device to have enhanced resistance to impacts, falls and water ingress. With HD+ Safety Shell the HD+ device reaches degree IP42. Thanks to the material and ergonomic shape it makes gripping the HD+ device easier making it even easier to handle when used in emergency situations. -
Page 24: Bluetooth Functions
INTRODUCTION The device function consists of acquiring and wirelessly transmitting ECG signal for displaying, processing and presenting ECG signal for the purpose of supporting the diagnose of patient conditions. The device does not store nor does associate patient identification data to the acquired signal, nor does it perform analysis on such signal. -
Page 25: Bluetooth Intended Use With Respect To Wireless Emitters
INTRODUCTION Rated power: 3.46 dBm Receiver sensitivity: -86 dBm Frequency: 2402 – 2480 MHz N° of channels: 79 Channel separation: 1 MHz Channel bandwidth: 0,871201157 MHz Range: 30 meters line-of-sight Integrated chip antenna ... - Page 26 INTRODUCTION...
-
Page 27: Preparation For Use
Through a Bluetooth connection, the HD+ transmits the acquired electrocardiographic signals in real-time to a computer where one of the compatible CARDIOLINE software is installed. Refer to the user manual of the individual software for a complete guide to the HD+ connection. - Page 28 PREPARATION FOR USE...
-
Page 29: Execution Of The Examination
EXECUTION OF THE EXAMINATION EXECUTION OF THE EXAMINATION 7.1. Preparing the patient Ensure that the patient fully understands the procedure and knows what to expect before connecting the electrodes. Privacy is very important to allow the patient to be relaxed. ... - Page 30 EXECUTION OF THE EXAMINATION 2. Position the electrodes on the flat and fleshy parts of the arms and legs. 3. If a limb is not available, position the electrodes on a blood-supplied stump. 4. Apply the electrodes on the skin. Test the correct adherence, and therefore the good contact, by pulling the electrode.
-
Page 31: Ecg Acquisition
EXECUTION OF THE EXAMINATION Reference table for the connection to the patient IEC lead AAMI lead Position Fourth intercostal space to the right of the sternum. Fourth intercostal space to the left Yellow Yellow of the sternum. Midway position between electrodes V2/C2 Green Green V4/C4. - Page 32 4. If the HD+ is properly connected to the receiver, the acquired ECG data is transmitted automatically. 5. Follow the instructions described in the user manual of the CARDIOLINE software installed on the receiver to acquire, print and save a tracing.
-
Page 33: Maintenance And Troubleshooting
MAINTENANCE AND TROUBLESHOOTING MAINTENANCE AND TROUBLESHOOTING 8.1. Precautions Switch the device off and remove the batteries before cleaning. Do not immerse the device in water. Do not use organic solvents, ammonia-based solutions or abrasive cleaning agents which could damage the surface of the device. -
Page 34: Cleaning The Device
MAINTENANCE AND TROUBLESHOOTING Do not immerse the cable terminations. Immersion may result in metal corrosion. Do not dry excessively or use forced heat to dry. ATTENTION: Avoid immersing the device in the liquid and do not apply a direct stream of liquid. Do not autoclave or steam clean. -
Page 35: Recommendations
8.9. Recommendations The device cannot be repaired. In the event of a failure, contact the Cardioline Authorised Service Centre to assess the extent of the failure and, possibly, to replace the device. In any case, where a non-compliant operation is suspected, the following procedures are recommended: ... - Page 36 MAINTENANCE AND TROUBLESHOOTING electrode must be replaced. Check if the terminal is connected or if the relevant RL or R RL or R anomaly electrode must be replaced. Check if the terminal is connected or if the relevant LA or F LA or F anomaly electrode must be replaced.
-
Page 37: Technical Specifications
Front-end performance ....ANSI/AAMI IEC 60601-2-25:2011 Data transfer ........Bluetooth 2.0+ EDR with “secure pairing” Lead-fail detection ......Independent for all leads Compatible devices ......Cardioline touchecg, Cardioline cubestress. Power supply ........2 AAA standard batteries Battery Duration ......More than 500 ECGs Dimensions ........ -
Page 38: Harmonised Standards Applied
TECHNICAL SPECIFICATIONS Environmental Specifications ... Storage and operating conditions: Ambient temperature: 0°C; +40°C Relative humidity: 25%; 95% Atmospheric pressure: 700 mbar; 1060 mbar Classification of Medical Devices ..IIa in compliance with the directive 93/42/EEC. 9.1. Harmonised standards applied STANDARD DESCRIPTION EN ISO 15223-1 Medical devices - Symbols to be used with medical device... -
Page 39: Accessories
TECHNICAL SPECIFICATIONS STANDARD DESCRIPTION equipment from working activities - Guidelines" 9.2. Accessories CODE DESCRIPTION HD+ Stress Belt (strap with bag for HD+) 67040211 HD+ Safety Shell (protective silicone shell for HD+) 67040212 63030105 Set of 4 colored peripheral ECG electrode clamps, Ag/Agcl 63030106 Set of 4 peripheral ECG electric clamp Ag/Agl 63030107... - Page 40 TECHNICAL SPECIFICATIONS...
-
Page 41: Warranty
Cardioline Spa declines all liability for any damage which may be caused, directly or indirectly, to persons or property as a consequence of non-compliance with all the prescriptions specified in the manual, especially warnings regarding installation, safety, use and maintenance of the equipment, as well as non-operation of the equipment. - Page 42 WARRANTY...
-
Page 43: Disposal
DISPOSAL DISPOSAL 11.1. Disposal of waste material The HD+ uses two AAA batteries and ECG disposable electrodes. They must be disposed of according to the following procedures: Battery: proper disposal or standard recycling Electrodes: normal waste 11.2. Disposal of the device Pursuant to Italian Legislative Decree no. - Page 48 Head Office and Production Via Linz, 151 38121 Trento Italy T. +39 0463 850125 F. +39 0463 850088 Sales Office: Via F.lli Bronzetti, 8 20129 Milan, Italy T. +39 02 94750470 F. +39 02 94750471...














Need help?
Do you have a question about the HD+ and is the answer not in the manual?
Questions and answers