Subscribe to Our Youtube Channel
Summary of Contents for Woodpecker ULTRASURGERY
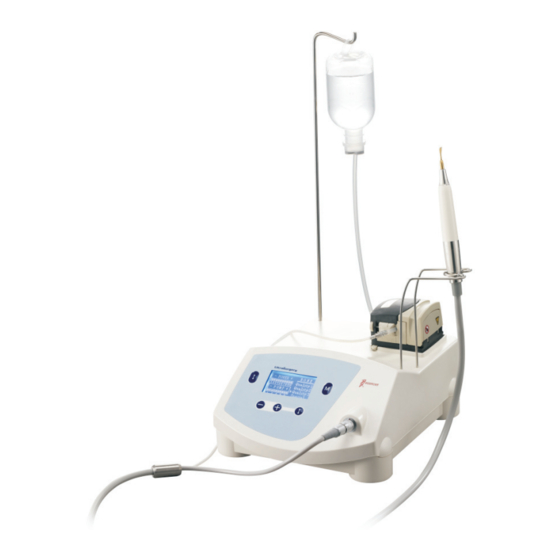
- Page 1 ULTRASURGERY MANUAL OF USE AND MAINTENANCE Industrial design patent No.: CN 200830301694.8...
-
Page 2: Table Of Contents
1.3 Intended use-------------------------------------------------------------------------2 1.4 Safety requirement-----------------------------------------------------------------2 2. Identification data-----------------------------------------------------------------------5 2.1 Identification data------------------------------------------------------------------5 2.2 Data plate of the device-----------------------------------------------------------5 2.3 Data plate of the ULTRASURGERY handpiece--------------------------------5 3. Testing of the device-------------------------------------------------------------------6 4. Delivery----------------------------------------------------------------------------------6 5. List of material included in the supply-----------------------------------------------6 6. Installation-------------------------------------------------------------------------------9 6.1 Safety requirements during Installation-----------------------------------------9... - Page 3 8.6 Autoclave sterilization of the torque wrench----------------------------------21 8.7 Autoclave sterilization of the peristaltic pump tube--------------------------21 8.8 Autoclave sterilization of the connection between cord and peristaltic pump tube connection------------------------------------------------------------------------21 8.9 Autoclave sterilization of the handpiece support------------------------------21 9. Regular maintenance------------------------------------------------------------------21 10. Replacement of the fuses------------------------------------------------------------22 11.
-
Page 4: Introduction
The user is not authorized to tamper with the equipment under any circumstances. If any problems are encountered, please contact a Woodpecker Service Centre. Any attempts on the part of the user or any unauthorized personnel to tamper with or alter the apparatus will invalidate the warranty and release the Manufacturers from any liability in respect of any harm or damage to persons or property. -
Page 5: Intended Use
Thanks to its controlled three-dimensional ultrasound oscillations, the original ULTRASURGERY technique rings in a new age for osteotomy and osteoplasty in Implantology, Periodontology, Endodontics and Orthodontic Surgery. Its main features are: Micrometric cutting: Maximum surgical precision and intra-operative sensibility; Selective cutting: Maximum safety for the soft tissues;... - Page 6 Danger: Contraindications. Do not use the ULTRASURGERY on patients with pace-makers or other implantable electronic devices. The same requirement applies also to the operator.
- Page 7 This equipment cannot function in places where there is an inflammable atmosphere. (anaesthetic mixture, oxygen, etc) Danger: Personnel injury. The foot switch of the ULTRASURGERY must not be activated when the door of the peristaltic pump open. (Fig.5—Ref.B).Moving parts could injure the operator. Danger: Contraindication.
-
Page 8: Identification Data
The data plate is on the rear of the device. The remaining data are included in this manual (see point 15). Fig.1 2.3 Data plate of the scaler handpiece The serial number of the Ultrasurgery handpiece is engraved on the ring nut (Fig.2). Fig.2... -
Page 9: Testing Of The Device
2.4 Data plate of the foot switch Fig.3 3. Testing of the device All the devices are checked and tested by Woodpecker completely, including all the parts. When testing, all the parts will work in intermittent operation. The test emphasized that all the problems are from the failure parts. - Page 10 Name Quality Torque wrench Fig.4—Ref.C ULTRASURGERY Fig.4—Ref.D handpiece complete with cord Connection for the cord Fig.4—Ref.E and tube of the peristaltic pump Connector of handpiece Fig.4—Ref.F Plug of Footswitch Fig.4—Ref.G Footswitch Fig.4—Ref.H Tip Holder Marked on the packing Fig.4—Ref.I list Marked on the packing Fig.4—Ref.J...
- Page 11 Fig.4...
-
Page 12: Installation
Applied parts : Handpiece , tips . 6. Installation 6.1 Safety requirements during Installation Danger: The wiring system of the premises where the apparatus is installed and used must comply with the applicable standards and the relevant electrical safety requirements. Danger: Do not install the apparatus in places where there is a risk of explosion. -
Page 13: Initial Installation
Close the door completely(Fig.5—Ref.C). Danger: personnel injury. The footswitch of the ULTRASURGERY must not be activated when the door of the peristaltic pump open. (Fig.5—Ref.B).Moving parts could injure the operator. 6.3.2 Insert the rod for supporting the bag into the holes provided for it (Fig.6—... - Page 14 (Fig.6—Ref.E) and then into the power outlet; 6.3.5 Insert the handpiece support into the two holes provided for it (Fig.6—Ref. 6.3.6 Insert the tube of ULTRASURGERY cord to the cord connector on the device (Fig.4—Ref.P); 6.3.7 Put the handpiece on the support (Fig.4—Ref.O);...
-
Page 15: Controls
Fig.8 7. Controls 7.1 Description of the controls This section illustrates the parts of the front panel of the ULTRASURGERY unit, enabling the controls described in this manual to be located immediately. 7.1.1Description of bone function: Fig.9 7.1.2 Description of root function:... -
Page 16: Description Of The Display And Functions
7.1.3 Description of clean function: Fig.11 7.2 Description of the display and functions There are three functions of bone root, clean for this ULTRASURGERY. 7.2.1 BONE function (Fig.9) In bone function, the power model is unavailable, but we can adjust the water... -
Page 17: Safety Requirements During Use
25 seconds. 7.3 Safety requirements during use. Danger: Contraindications. Do not use the ULTRASURGERY on patients with pacemakers or other implantable electronic devices. This requirement also applies to the operator. Danger: Breakage and wear of the tips. -
Page 18: Protection Systems And Alarms
These are shown on the display, as follows: Warning code Possible Cause There is a signal transmission problem. Warn 01 Switch the device off and then on again, If the problem persists contact Woodpecker service centre. Warn 02 Tuning circuit not working properly. Handpiece failure. Warn 03 Fan failure. -
Page 19: Instruction For Use
Woodpecker service centre immediately. 7.5 Instruction for use 7.5.1 Open the air intake on the drip system; 7.5.2 Screw the chosen tip onto the ULTRASURGERY handpiece until it is flush against it; 7.5.3 To use the torque wrench correctly (Fig.8) proceed as follow;... -
Page 20: Rules For Keeping The Device In Proper Working Order
“+”/“ - ”(see7.1)to choose,, depending on the type of function that has been set. 7.6 Rules for keeping the device in proper working order 7.6.1 Check the state of wear of the tips periodically and replace any for which a drop in performance is noted;... -
Page 21: Cleaning, Disinfection And Sterilization
8. Cleaning, Disinfection and Sterilization. 8.1CLEAN function—Cleaning of the liquid circuit Warning: Failure to carry out cleaning of the tubes will lead to crystallization of salts that can seriously damage the equipment. Warning: Handpiece and cord can’t be detached. a) Change the bag containing water (demineralized water is recommended); b) Check whether the water system is connected correctly;... -
Page 22: Sterilization Procedure
the disinfectant solution. Allow the disinfectant solution to dry in the air before using the apparatus. NOTE: Water-based disinfecting solutions with a neutral Ph are highly recommended, some alcohol-based disinfecting solutions may be harmful and discolour or otherwise damage plastic materials. 8.3 Sterilization procedure Warning: Carry out sterilization using only a steam autoclave. -
Page 23: Autoclave Sterilization Of The Tips
and of the cord must be dry; At the end of the sterilization cycle and before connecting the handpiece to the cord, make sure that the electric contacts of both connectors are completely dry, if necessary, dry the contacts by blowing air on to them with the syringe. Warning: After completing the sterilization cycle, allow the handpiece to dry completely before using it. -
Page 24: Autoclave Sterilization Of The Torque Wrench
8.5.5 Autoclave sterilizes the tips. 8.6 Autoclave sterilization of the wrench for tightening the tips 8.6.1 Clean the wrench; 8.6.2 Disinfect the wrench with a mild disinfectant solution having a neutral Ph 7 and dry it thoroughly; 8.6.3 Seal the wrench inside an individual disposable bag; 8.6.4 Autoclave sterilizes the wrench. -
Page 25: Replacement Of The Fuses
5 minutes per time. 9.5 disconnect the device from the power mains. Danger: Check regularly that the power cable is intact, if it is damaged, replace it with an Woodpecker spare. 10. Replacement of the fuses Danger: Switch off the apparatus. -
Page 26: Disposal Procedures And Precautions
10.4 Put the compartment back into place (Fig.12-Ref.B). Fig.12 11. Disposal procedures and precautions Danger: Hospital waste Treat the following items as hospital waste - Tips, when worn or broken. - Irrigator, after each treatment. - Tube of the peristaltic pump, after 8 sterilizing cycles. - Torque wrench for tightening tips, when worn or broken. -
Page 27: Symbols
preparation of the site of an implant. 12.3 Blunt tips Blunt tips are used for separating the soft tissues, for example for detaching schneider’s membrane or for lateralizing nerves. In periodontology, these tips are used to smooth the root surfaces. 13. -
Page 28: Troubleshooting
Degree of protection against the impact of continuous diving Manufacturer Caution mechanical injury Protective earthing Consult the accompanying documents Authorised Representative in the EUROPEAN COMMUNITY Appliance compliance WEEE directive Serial number Got the quality management system certification and CE certification issued by TüV Rheinland 14. - Page 29 The footswitch will not Contact the nearest properly. work. dealer or authorized Woodpecker service centre. A faint whistle can be The tip is not correctly Unscrew the tip and heard coming from the tightened onto the...
- Page 30 WARN appears Lack of continuity of a lead Contact the nearest on the display. in the cord. dealer or authorized Woodpecker service centre. Handpiece failure. Contact the nearest dealer or authorized Woodpecker service centre. Malfunctioning of the Contact the nearest tuning circuit.
-
Page 31: Technical Data
The device is working Too much pressure by the Check that the tube in properly, but the pump is impeller on the tube in the the peristaltic pump has being forced. peristaltic pump. been correctly inserted. The pump is running The door of the peristaltic Make sure that the door correctly but when it... - Page 32 IPX8 (footswitch) 15.3 Device for intermittent operation: 60s ON, 10s OFF 15.4 Power-supply voltage: ~ 220±22V 50Hz/60Hz 360mA (Max) 15.5 Fuses: 2×0.5AT 250V 15.6 Working frequency: 24kHz ~ 29.5kHz 15.7 Flow: 25 ~ 100ml/m 15.8 Protection systems and tripping time of the APC: No handpiece connected: 10ms Cord interrupted: 10ms Tips broken or not correctly tightened: <...
-
Page 33: After Service
15.14 Size of main unit: 330 mm×254 mm×167mm 15.15 Weight of main unit: 3.8kg 15.16 Type of protection against electric shock: Class Ⅰ equipment 15.17 Degree of protection against electric shock: Type B applied part 16. After service We offer one year, free repair to the equipment according to the warranty card. The repair of the equipment should be carried out by our professional technician. -
Page 34: Declaration Of Conformity
20. Declaration of conformity 20.1 Product conformity the following standards: EN 60601-1:2006 EN 60601-1-2:2007 EN 60601-1-4:1996 EN 60601-1-6:2007 EN 61847:1998 EN 980:2008 EN 1041:2008 EN ISO 14971:2009 EN ISO 7405:2008 EN ISO 17664:2004 EN ISO 17665-1:2006 EN ISO 10993-1:2009 EN ISO 10993-5:2009 EN ISO 10993-10:2010 20.2 Declaration of conformity- EMC... -
Page 37: Guarantee
21.2 WOODPECKER guarantees its products, purchased new from a WOODPECKER dealer or importer, to be free from manufacturing or material defects for: -1 YEAR from the date of purchase for the device;... - Page 38 The warranty is valid only if the warranty slip enclosed with the product has been completed in full and returned to us or, if appropriate, to your WOODPECKER dealer or importer within 20 days from the date of purchase, as proven by the consignment note/invoice issued by the dealer/importer.
-
Page 39: Statement
In the event of failure due to accidents or improper use, or if the warranty has lapsed, repairs to WOODPECKER produces will be charged on the basis of the actual cost of the materials and labour required fro such repairs. - Page 40 GUILIN WOODPECKER MEDICAL INSTRUMENT CO., LTD. The industrial design, inner structure, etc, have claimed for several patents by WOODPECKER, any copy or fake product must take legal responsibilities.
- Page 41 ZMN/WI-04-527 2.3Edition...






Need help?
Do you have a question about the ULTRASURGERY and is the answer not in the manual?
Questions and answers