Summary of Contents for Pari LC SPRINT COMPACT
- Page 1 Instructions for use ® PARI LC SPRINT COMPACT Model: PARI LC SPRINT COMPACT (Type 023) Nebulisers for PARI lnhalation systems...
- Page 2 These instructions for use describe the components of PARI products and optional ac- cessories. For this reason, these instructions for use also describe and illustrate features not present in your PARI product because they are, for instance, country-specific and/or op- tional. When using the systems, products and functions, the applicable country-specific regu- lations must be observed.
- Page 3 TABLE OF CONTENTS IMPORTANT INFORMATION ..............5 Intended purpose.....................5 Indication .........................5 Contraindication.......................6 Labelling ........................6 Safety and warning instructions................7 PRODUCT DESCRIPTION ..............10 Components ......................10 Overview and designations ...................10 Product variants.....................11 Product combinations....................11 Description of function...................11 Material information....................12 Service life......................12 USE......................13 Preparing for treatment..................13 Performing treatment.....................15 Ending the treatment .....................17...
- Page 4 REPROCESSING IN PROFESSIONAL HEALTH INSTITUTIONS ..... 23 Reprocessing cycles....................23 Processing limits....................23 Nebuliser .......................24 Connection tubing....................30 Visual inspection and storage................30 TROUBLESHOOTING................31 TECHNICAL DATA ................. 32 General nebuliser data ..................32 Aerosol characteristics according to ISO 27427............32 FURTHER INFORMATION ..............33...
- Page 5 The PARI CENTRAL is intended for the connection with the central gas supply sys- tem. This PARI product can be used in a home environment, as well as in professional health in- stitutions. When used in a home environment, this PARI product is intended for single-pa- tient use only (no patient change).
- Page 6 1.3 Contraindication There are no contraindications known to PARI GmbH. 1.4 Labelling The following symbols can be found on the product and/or the packaging: Medical device Unique Device Identifier (UDI) Legal manufacturer Date of manufacture Item no. Production batch number, lot number This product conforms to the EU Medical Device Regulation 2017/745.
- Page 7 The user must follow these in order to guarantee safe operation of this PARI product. This PARI product must be used only as described in these instructions for use. The instructions for use of the compressor and accessories used and the information for use of the inhalation solution used must also be followed.
- Page 8 General If non-approved solutions or suspensions are used for nebulisation, then nebuliser aerosol characteristics may differ from the information provided by the manufacturer. This product is not suitable for use in an anaesthetic breathing system or a ventilator breath- ing system. Tracheotomised patients cannot inhale using a mouthpiece.
- Page 9 Hygiene Observe the following hygiene instructions: – Do not use product components unless they have been thoroughly cleaned and dried. Contamination and residual moisture encourage the growth of bacteria, which increases the risk of infection. – Before every use and reprocessing cycle, wash your hands thoroughly. –...
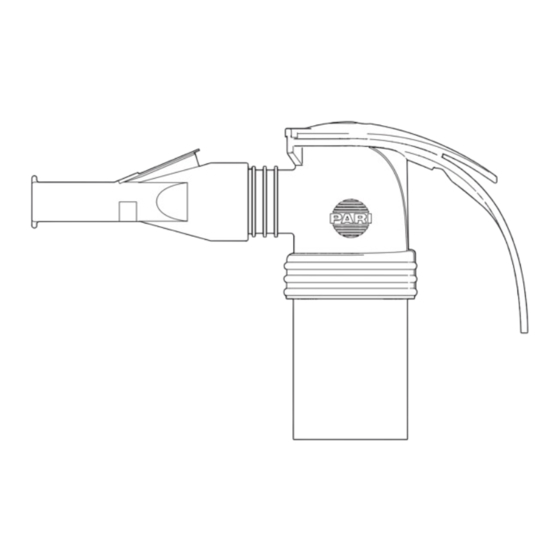
- Page 10 PRODUCT DESCRIPTION 2.1 Components Please refer to the package for information on the supplied components. 2.2 Overview and designations 1 Nebuliser Nebuliser upper part Nozzle attachment Nebuliser lower part LC interrupter Mouthpiece Connection tubing 2 Air filter for compressor...
- Page 11 – With mouthpiece for treatment of the airways in adults and children aged 4 years and older. – With PARI baby mask soft for the treatment of babies aged between 6 months and 3 years. – With PARI child mask for the treatment of children aged 4 years and above.
- Page 12 Nebuliser, connection tubing Professional environment [see: Processing limits, and accessories page 23] When the expected operating life has been reached, replace the affected component. Nebuliser replacement sets (nebuliser including connection tubing) or PARI spare filter for PARI compressors are available.
- Page 13 • The PARI CENTRAL O2 is no longer sold. • If you use a PARI CENTRAL O2 which is still on the market, perform the treatment us- ing oxygen only after consultation with, and under the supervision of, a professional.
- Page 14 • Attach the connection tubing to the nebuliser. • Attach the LC interrupter to the nebuliser. • Insert the connection tubing in the air inlet on the side of the LC interrupter. Using the mouthpiece • Fit the mouthpiece onto the nebuliser. Using accessories Information on assembling accessories is included in the instructions for use of the respect- ive accessory...
- Page 15 Filling the nebuliser NOTE Nebuliser lid might break off If the cap is twisted in the wrong direction, it may break off. The nebuliser will then be un- usable and irreparable. • Never move the lid except in the direction allowed by the hinge. •...
- Page 16 • DANGER! Life-threatening situation if tubes are mixed up! If tubing systems for other devices are present close by (e.g., for infusions), check carefully to ensure that the other end of the connection tubing connected to the compressor is connected to the nebuliser.
- Page 17 Inhaling with the mouthpiece • Sit in an upright position and relax. • Hold the mouthpiece between your teeth and enclose it with your lips. • Breathe in as slowly and deeply as possible through the mouthpiece, and out again calmly. •...
- Page 18 REPROCESSING IN A HOME ENVIRONMENT The product components must be cleaned thoroughly immediately after each use, and disin- fected once a week. The connection tubing cannot be cleaned or disinfected. Dry the connection tubing after each use [see: Care of the connection tube, page 22]. The maximum operating life of the connection tubing is 1 year.
- Page 19 Manual cleaning EQUIPMENT: – Drinking water temperature of about 40 °C – Standard commercial washing-up liquid – Receptacle with at least 3 l capacity PROCEDURE: • Unless otherwise specified by the manufacturer, add about 1 teaspoonful washing-up liquid to 3 l warm drinking water. •...
- Page 20 4.5 Disinfecting Disinfect all individual parts after cleaning. Only components that have been cleaned can be disinfected effectively. The validated disinfection procedures are described below. In boiling water EQUIPMENT: – Clean cooking pot – Drinking water PROCEDURE: CAUTION Risk of infection due to moisture Moisture encourages the growth of bacteria.
- Page 21 Using a standard thermal disinfector for baby bottles (not a microwave oven) EQUIPMENT: – Thermal disinfector with a runtime of at least 6 minutes. PROCEDURE: CAUTION Risk of infection due to inadequate disinfection Inadequate disinfection encourages the growth of bacteria and thus increases the risk of infection.
- Page 22 4.6 Care of the connection tube Dry the connection tube after every inhalation session: • Connect the connection tube to the compressor. • Switch on your compressor. • Let the compressor continue to run until all the moisture in the tube has been removed. 4.7 Inspecting Inspect all product components after each cleaning and disinfection.
- Page 23 REPROCESSING IN PROFESSIONAL HEALTH INSTITUTIONS Dry the connection tubing after each use [see: Connection tubing, page 30]. 5.1 Reprocessing cycles Single patient use Nebuliser excluding connection – Clean immediately after every use tubing and accessories (e.g. – Disinfect once per week masks) Before a change of patients Nebuliser without connection tubing –...
- Page 24 • Replace the connection tubing or carry out mechanical cleaning and disinfection of the connection tubing [see: Connection tubing, page 30]. All components of a PARI nebuliser and the PARI accessories used can be cleaned, disin- fected and sterilised according to the procedures described below.
- Page 25 Cleaning and disinfecting Please observe the instructions for use for the chemicals used. Manual cleaning EQUIPMENT: The method has been validated in Europe using: – pH-neutral cleaning agent: ® Bode Bomix plus (concentration: 0.1%) – Application time: 10 minutes PROCEDURE: CAUTION Risk of infection due to growth of bacteria Inadequate disinfection encourages the growth of bacteria and thus increases the risk of infection.
- Page 26 Cleaning with disinfection To ensure safety when handling chemicals, follow the instructions for use of the disinfectant. Mechanical cleaning EQUIPMENT: with disinfection: The method has been validated in Europe using: – Alkaline cleaning agent: ® Dr. Weigert neodisher MediClean forte (concentration: 0.5%) –...
- Page 27 Chemical cleaning EQUIPMENT: with disinfection: The method has been validated in Europe using: ® – Aldehyde-free instrument disinfectant: Bode Bomix plus (concen- tration: 2%) Active agent basis: Quaternary ammonium compound – Application time: 5 minutes PROCEDURE: • Clean and disinfect the individual parts in a single work step with a solution prepared according to the manufacturer’s instructions.
- Page 28 • Rinse the product thoroughly to ensure that no residues of the disinfectant remain on the PARI product. • Rinse off all parts thoroughly in running water at about 15 °C for 3 minutes. • Dispose of the used solution. Unless otherwise specified by the manufacturer of the disin- fectant, the diluted solution can be disposed of down the drain.
- Page 29 Sterilising CAUTION Risk of infection by residual germs If there is dirt on the parts, germs capable of reproduction may remain despite the steril- isation process. As a result, there is a danger of infection. • Clean, disinfect, and dry all parts thoroughly before sterilising. •...
- Page 30 5.4 Connection tubing Mechanical cleaning and disinfecting EQUIPMENT: The method has been validated in Europe using: ® – Alkaline cleaning agent: Dr. Weigert neodisher MediClean forte ® – Neutralising agent: Dr. Weigert neodisher – Cleaning and disinfection device: RDG G7836 CD (Miele) (conforming to DIN EN ISO 15883) –...
- Page 31 TROUBLESHOOTING Contact the manufacturer or distributor: – in the event of faults that are not listed in this chapter. – if the suggested procedure does not correct the fault. Fault Possible cause Remedy No aerosol is com- The nebuliser nozzle is Clean the nebuliser.
- Page 32 TECHNICAL DATA 7.1 General nebuliser data Size 10 cm × 10 cm × 4 cm Weight 31 g to 33 g Operating gases Air, oxygen Minimum compressor flow 3.0 l/min. Minimum operating pressure 0.5 bar / 50 kPa Maximum compressor flow 6.0 l/min. Maximum operating pressure 2.0 bar / 200 kPa Minimum fill volume 2 ml Maximum fill volume 8 ml...
- Page 33 FURTHER INFORMATION All product components may be disposed of with normal domestic waste. The country-spe- cific disposal regulations must be observed. 6) Operation with PARI COMPACT2 compressor (Type 152). 7) MMAD = Mass Median Aerodynamic Diameter 8) GSD = Geometric Standard Deviation...
- Page 36 PARI GmbH Spezialisten für effektive Inhalation Moosstraße 3 82319 Starnberg • GERMANY info@pari.de • www.pari.com ©2023 PARI GmbH Spezialisten für effektive Inhalation, 023D2301-A en 2023-08-01...















Need help?
Do you have a question about the LC SPRINT COMPACT and is the answer not in the manual?
Questions and answers